| Pages:
1
2
3 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
If the problem is that the synthesis gave you 1g instead of the expected 37, then I suggest you check your procedures and starting materials . . .
|
|
|
kiko
Harmless

Posts: 6
Registered: 3-11-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | If the problem is that the synthesis gave you 1g instead of the expected 37, then I suggest you check your procedures and starting materials . . .
|
the procedure is as in the paper FOX-& A new insensitive high explosive by DSTO??
i dont know if the problem in the dryness step of in the filter paper or in what????
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Right now the way you're describing your problem amounts to gibberish. You haven't shared any details on the procedure and materials and reagents YOU
used, no details on any observations during preparation, and nothing on the final product. All you've said is "I looked at this paper and then
everything went wrong," with considerably less intelligibility and giant strings of punctuation. You can't seriously expect any answers...
I weep at the sight of flaming acetic anhydride.
|
|
|
AH-Poster
Harmless

Posts: 9
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
Just a quick thought on a shortcut to Fox-7,
starting with 1,1,1-trinitro ethane
see figure 1.69 in franklyn's post on page 4 of the topic "New Energetic Materials - Current Research".
1,1,1-trinitro ethane reacts with KOH to form
1,1-dinitroethylene and KNO2. This can be inferred from the description of the picture, and this was specifically stated in one of the articles from
Journal Russian Chemical Bulletin
S. S. Novikov1, I. S. Korsakova1 and K. K. Babievskii
N. D. Zelinskii Institute of Organic Chemistry Academy of Sciences USSR, USSR
(they authored several articles about similar compounds)
A similar reaction, except the base pulling out an HCl instead of a HNO2 can be seen on wikipedia,
http://en.wikipedia.org/wiki/1,1-Dichloroethene
Obviously dinitroethylene can be expected to react with chlorine gas, to generate 1,1-dinitro,2,2-dichloro ethylene,
and from here simply condense with anhydrous ammonia.
I cannot find a specific reference for dichloro ethylene reacting with amines or ammonia, likely because (with the nitro groups not present) the
product would be a gunky mess. Basically an imine of acetaldehyde that would be unstable because of the acidity of the HCl.
It is probably easer to make 1,1,1-trinitroethane than the
normal imidazolidinedione precursor. For example, from ethyl nitrolic acid CH3C(=NOH)NO2 reacting with more NO2.
A google books "organic chemistry of explosives" claims that
the double nitrolate of ethane can be nitrated to hexanitroethane by being treated with concentrated H2SO4 and HNO3 dissolved in CH2Cl2 (92% yield).
Obviously this would be unsuitable for ethyl nitrolic acid, since the methyl group would get oxidized, but perhaps using NO2, which is known to form
psuedonitrosoles from oximes. There are references on my site about NO2 oxidizing nitroso groups or nitrites. For specifics about ethyl nitrolic acid
there is information already posted on this site for ethyl nitrolic acid, see A. Hantzsch and O. Graul (Ber. 1898, 31, p. 2854),
and post #13 (which is subtitled 1,1,1-trinitroethane)
in the topic "1,2,2 trinitro propane". Ethyl nitrolic acid results from nitric acid nitration of acetone. See the topic "Silver salt of
acetylmethylnitrolic acid".
The article at the bottom of this page
https://sites.google.com/site/energeticchemical/5-dinitromet...
discusses an even more energetic compound (which can form salts) which can be made by diazotizing the amines of Fox-7 into a tetrazole ring. This
might not be as powerful as DTTO, but at least the route is straightforward.
[Edited on 6-11-2010 by AH-Poster]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Energeticchemical is your site Anders?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Easy synthesis for Fox-7 ?
This is just an idea for a procedure to make the energetic compound, Fox-7, using readily obtainable precursors.
(1) oxidize ethylene glycol to glycolaldehyde
(the glycolaldehyde does not need to be separated from the unreacted ethylene glycol)
Glycolaldehyde is also common and easily obtainable from chemical supplies, if you find the oxidation procedure to be too messy or inconvenient.
(2) reflux the glycolaldehyde with ammonia solution and acetic acid. This will form 2-methyl imidazole.

A similar condensation cyclisation has already been described in the literature: "Imidazoles from ammoniacal solutions of glycolaldehyde and
hydroxypyruvaldehyde" M.R. Grimmett and E.L. Richards
(3) Perform a nitration on the 2-methyl imidazole using mixed acids. This should yield 2-dinitromethyl-4,5-dinitro imidazole.
(4) Dilute with water and separate out the nitrated product.
Then react with concentrated ammonium hydroxide. The 2-dinitromethyl-4,5-dinitro imidazole will hydrolyse into Fox-7, ammonium
nitrite, and an aldehyde-ammonia condensation product.
So basically, the only precursors needed would be:
anti-freeze
KMnO4
white vinegar
98%conc H2SO4
KNO3 or 70%conc HNO3
household cleaning ammonia
What do you think? Any comments? Obviously it has not been tested, but it seems relatively straightforward.
(you might also see my other post about using Fox-7 to make an even more powerful explosive)
Quote: Originally posted by AndersHoveland  |
click here ^ to be directed to the other post
Reacting 1,1-diamino-2,2-dinitroethylene (Fox-7) with NaOCl (bleach) might form 4,4’-Dinitro-3,3’-diazenofuroxan (DDF).
|
[Edited on 17-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I think you should try it yourself. I also think that if you do so, you should write it
up for others to examine.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
+1
Work on a gram(s) level & document the temps and idiosyncrasies.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I don't think you can nitrate that methyl group to a dinitromethyl. There seems to be solid references to back that up. There are other ways to go
about it though. Have a look at this:
http://docs.google.com/viewer?a=v&q=cache:z_lnmS1fAe8J:j...
Edit
Yes, you're right, I misread that.
[Edited on 17-8-2011 by 497]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | I don't think you can nitrate that methyl group to a dinitromethyl. There seems to be solid references to back that up. There are other ways to go
about it though. Have a look at this:
|
That information actually mentions that Fox-7 has been prepared from 2-methylimidazole.
This would seem to contradict the information in your link:
http://books.google.com/books?id=xF5yUHI0m4QC&pg=PA50&am...
That article that you cited essentially says that 2-methyl-4-nitro-imidazole cannot have its methyl group nitrated. The nitration of the methyl group
proceeds through an unsaturated tautomer, which is favorable under acidic conditions. Apparently, this tautomer cannot form if there are nitro groups
already on the ring. Indeed, both 4-nitro-, and 4,5-dinitro-, 2-methylimidazole also form as byproducts during the nitration of 2-methylimidazole, as
their methyl group has apparently become "deactivated".
2-methylimidazole was simultaneously nitrated and oxidized to 2-[dinitromethylene]-4,5-imidazolidinedione, which was then hydrolysed to Fox-7.
[Edited on 17-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I don't know if this has been mentioned or not but I did notice another very OTC route the 2-methylimidazole that has literature to back it up every
step of the way. If Anders' above route works, it would surely be easier, but I was unable to find anything solid to confirm that it works. So here it
is:
Alanine + TCCA --H2O/NH3--> acetonitrile 90% yield (or buy it if you can)
Acetonitrile + EtOH + HCl --> iminoether (not isolated)
Iminoether + NH3 --EtOH--> acetamidine 90% yield
Acetamidine + glycolaldehyde/chloroacetaldehyde --> 2-methylimidazole 80-90% yield?
Not too tough... If you have easy access to acetonitrile and glycolaldehyde it would be downright simple.
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
Edit
Does anyone know if a 4 alkyl substituent would screw up the nitration? If it didn't, things think chloroacetone could be used instead of
glycolaldehyde. Either way the yields on the ring closure reaction should be good according to
http://www.orgsyn.org/orgsyn/prep.asp?prep=v81p0105
[Edited on 17-8-2011 by 497]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  |
I was unable to find anything solid to confirm that it works.
Does anyone know if a 4 alkyl substituent would screw up the nitration? If it didn't, things think chloroacetone could be used instead of
glycolaldehyde. Either way the yields on the ring closure reaction should be good according to
http://www.orgsyn.org/orgsyn/prep.asp?prep=v81p0105
|
"A solution of glycolaldehyde in concentrated ammonia solution became straw- coloured after 8 weeks at room temperature. From the resulting mixture
imidazole and 2-hydroxyrnethylimidazole have been isolated."
M. R. Grimmett and E. L. Richards, Australian Journal of Chemistry
There is a biref mention to it in this article:
"The formation of imidazole from glycolaldehyde and concentrated ammonia..."
http://www.springerlink.com/content/x9512th9wuu54125/
It seems to be a variation of the Radziszewski reaction.
Apparently amidines (such as acetamidine) readily reacts with α-haloketones (such as chloroacetone) to yield imidazoles.
Fairly certain that one would not need to go to the trouble of preparing acetamidine. For the sythesis of the explosive NTO, for example, formic acid
is refluxed with semicarbazide to yield 1,2,4-triazole-3-one. Here is the specifics of the procedure if you are interested:
"47 g of semicarbazide and 115 g of 98% formic acid and 200 mL of ethanol is refluxed (heated to 100 degC and stirred) for 1 hour and then the excess
of formic acid and the ethanol are distilled off. 58 g of white solid formilsemicarbazide crystallized, with a melting point of 129 degC. This is then
dissolved in 150 mL of formic acid and refluxed for 1 hour. The excess of formic acid is distilled off. 25 g of a white solid are obtained which is
mostly 1,2,4 triazole-3-oxide." (it can then be nitrated with boiling 70%conc HNO3 to the NTO)
Unfortunately, it is quite probable that a 4-methyl substituent would mess up the reaction. The same thing that is supposed to happen to the methyl
group in the 2-position will also/or instead happen to the new alkyl group. The yields would likely be reduced by half, and it could become much more
complicated to separate out the final Fox-7 from structurally similar byproducts.
Some people have previously speculated about the reaction between 1,1-dinitro,2,2-dichloro ethylene and anhydrous ammonia. Fox-7 is not formed from
this reaction. Unfortunately, the condensation only yields the ammonium salt of dinitroacetonitrile:
NH4(+) NΞC–C(NO2)=NO2(-)
sorry, unable to find the reference now
[Edited on 19-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
But 1,1-dinitro-2,2-dichloroethylene reacts with urea or amides to yield related to FOX-7 derivatives...
Also trichlorethylene reacts with HNO3 69% to yield, upon standing (or boiling), 1-nitro-1,2,2-trichlorethylene...the yellow-orange liquid is very
pungent and is to my feeling as poisonous as chloropicrin (trichloronitromethane)... It displays the same kind of reactivity towards proteins as the
previously mentionned dinitrodichloroethylene 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
But can you selectively oxidize just to the glycolaldehyde using KMnO4, as you suggest? Seems like under basic conditions you end up going all the way
to the acid (oxalic or maybe glycolic), whereas under acid conditions you just cleave the diol. The German wikipedia page on glycolaldehyde (http://de.wikipedia.org/wiki/Glycolaldehyd) suggests that careful oxidation using Fe(II)SO4 and hydrogen peroxide will do the trick, but the
provided reference is a book I don't have access to, so it's hard to say just how finicky the conditions implied by 'careful' are...
[Edited on 21-8-2011 by bbartlog]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It is not selective oxidation, just limited oxidation. For best yields use a large excess of ethylene glycol.
http://www.erowid.org/archive/rhodium/chemistry/acetaldehyde...
Unfortunately, preparing glyoxal is not quite as easy.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
FOX-7 an expensive explosive!
"The estimated material cost for producing unpurified FOX-7
on a laboratory scale at DSTO vie the preferred FOI Method 2
was calculated at ca. AU$300/100g."
Attachment: Fox 7 page 6.pdf (107kB)
This file has been downloaded 758 times
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Not really. The cost of Fox-7 is estimated to only be around 0.50 USD per gram (or 500/kg) when the production is scaled up. In any case, even TNAZ is
not really so expensive in comparison to the cost of typical weapon delivery systems.
If the missile costs 1 million USD, even 10,000 dollars should not be considered overly expensive for the explosive filling, if weight can be reduced
or performance increased by a small margin. A 10% reduction in weight of the explosive, for example, could lead to savings of tens of thousands of
dollars for a smaller delivery system.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | Not really. The cost of Fox-7 is estimated to only be around 0.50 USD per gram (or 500/kg) when the production is scaled up. In any case, even TNAZ is
not really so expensive in comparison to the cost of typical weapon delivery systems.
If the missile costs 1 million USD, even 10,000 dollars should not be considered overly expensive for the explosive filling, if weight can be reduced
or performance increased by a small margin. A 10% reduction in weight of the explosive, for example, could lead to savings of tens of thousands of
dollars for a smaller delivery system. |
If [Insert quote about the Queen here --> ]
Seems to be that its primary advantage is that it is insensitive.
Ref attached PDF.
Actually its too large try these —
http://www.dtic.mil/ndia/2009insensitive/5AOEstmark.pdf
And —
Accession Number : ADA399359
Title : FOX-7 - A New Insensitive Explosive
Descriptive Note : Technical rept.
Corporate Author : DEFENCE SCIENCE AND TECHNOLOGY ORGANISATION VICTORIA (AUSTRALIA) AERONAUTICAL AND MARITIME RESEARCH LAB
Personal Author(s) : Lochert, Ian J.
Handle / proxy Url : http://handle.dtic.mil/100.2/ADA399359
Report Date : NOV 2001
Pagination or Media Count : 36
Abstract : 1,1-Diamino-2,2-dinitroethene (FOX-7) is an
energetic material developed by FOI Sweden in the late 1990s.
Theoretical studies have predicted that the performance of FOX
-7 should exceed that of RDX. FOX-7 is also significantly less
sensitive than RDX, particularly to impact and frictions stimuli.
Consequently, FOX-7 is an attractive ingredient for application
in high performance, IM-compliant explosive compositions.
AMRL evaluation of FOX-7 has focused on the synthesis,
hazards characteristion and performance studies. Aspects of
these research areas, including preliminary experimental data
that validates the theoretical performance values, are detailed.
By da there is a European and World Patent on Fox-7 e.g.,
WO 9903818. If I really cared how it is made I would look them
up.
|
|
|
Francis0126
Harmless

Posts: 4
Registered: 16-8-2011
Location: China Nanjing
Member Is Offline
Mood: It is hard to say ~~
|
|
Can you image the chemical behavior about Fox-7? 
I am here now,energetic materials!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Also found this in an online search:
| Quote: |
4-Methylimidazole can be manufactured through the reaction of hydroxy-acetone with formamide and liquid ammonia.
|
To make Fox-7, of course, one would need to 2-methylimidazole, not 4-Methylimidazole. But this does help show how easy simply precursors can condense
to form imidazoles.
You might see "The Structure and Chemical Bond of FOX-7: The AIM Analysis and Vibrational Normal Modes", Jamshid Najafpour, Iranian Journal Chemical
Engineering, p118
The Fox-7 molecule is aromatic, the amino groups are electron-donating to the nitro groups. The carbon-carbon bond is not a true double bond in
character.
(NH2)2C=C(NO2)2
[Edited on 22-2-2012 by AndersHoveland]
|
|
|
Francis0126
Harmless

Posts: 4
Registered: 16-8-2011
Location: China Nanjing
Member Is Offline
Mood: It is hard to say ~~
|
|
thank you for supporting,i want to do more reasearch about the fox-7 while its simple structure. it just like TATB ,hydrogen bonding plays the
key role for its insenstive. it is very interesting to combine two fox-7 together.what we can do for improving the insenstive or its detonation
velocity? just from the main structure of fox-7,may be some other derivatives? Quote: Originally posted by AndersHoveland  | Also found this in an online search:
| Quote: |
4-Methylimidazole can be manufactured through the reaction of hydroxy-acetone with formamide and liquid ammonia.
|
To make Fox-7, of course, one would need to 2-methylimidazole, not 4-Methylimidazole. But this does help show how easy simply precursors can condense
to form imidazoles.
You might see "The Structure and Chemical Bond of FOX-7: The AIM Analysis and Vibrational Normal Modes", Jamshid Najafpour, Iranian Journal Chemical
Engineering, p118
The Fox-7 molecule is aromatic, the amino groups are electron-donating to the nitro groups. The carbon-carbon bond is not a true double bond in
character.
(NH2)2C=C(NO2)2
[Edited on 22-2-2012 by AndersHoveland] |
I am here now,energetic materials!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
There is another derivitive of Fox-7, a very powerful one.
Some researchers have referred to it as "N2FOX-7". It is discussed on page 12 of the thread "5-ATZ(5-Aminotetrazole), the nitrotetrazolate ion and
friends" in this forum, the thirteenth post down. (or you can just click the link in the quote box below)
Quote: Originally posted by AndersHoveland  | I think the most promising energetic compound may be dinitromethyl tetrazole.
“Syntheis of 5-Dinitromethyltetrazole”, A. V. Shastin, B. L. Korsunskii, T. I. Godovikova, V. P. Lodygina. (2009) Russia
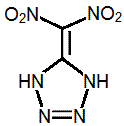
|
I made five posts about this compound in that thread, about the research, expected properties, and possible synthesis routes.
[Edited on 17-3-2012 by AndersHoveland]
|
|
|
Francis0126
Harmless

Posts: 4
Registered: 16-8-2011
Location: China Nanjing
Member Is Offline
Mood: It is hard to say ~~
|
|
Quote: Originally posted by AndersHoveland  | There is another derivitive of Fox-7, a very powerful one.
Some researchers have referred to it as "N2FOX-7". It is discussed on page 12 of the thread "5-ATZ(5-Aminotetrazole), the nitrotetrazolate ion and
friends" in this forum, the thirteenth post down. (or you can just click the link in the quote box below)
Quote: Originally posted by AndersHoveland  | I think the most promising energetic compound may be dinitromethyl tetrazole.
“Syntheis of 5-Dinitromethyltetrazole”, A. V. Shastin, B. L. Korsunskii, T. I. Godovikova, V. P. Lodygina. (2009) Russia
|
I made five posts about this compound in that thread, about the research, expected properties, and possible synthesis routes.
[Edited on 17-3-2012 by AndersHoveland] |
I have done some reasearch about the substance 5-Dinitromethyltetrazole , the outstanding work is the synthesis of the Dinitromethyltetrazole
salts"Energetic mono and dibasic 5-dinitromethyltetrazolates: synthesis,properties, and particle processing"published in 2007. How about the nitration
of dinitromethyltetrazole? Is it possible for the -NH to be -N-NO2?
I am here now,energetic materials!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I am not sure, but I think this would just form trinitronitromethyltetrazole, with the 3 nitro groups on the same carbon atom. Further nitration of
Fox-7, for example, is certainly possible, but much more difficult. Dinitromethyltetrazole would be even more resistant to nitration. It would
probably require N2O5 in HNO3. But I do not understand why one would nitrate it. Dinitromethyltetrazole probably already has a good sensititivity to
power ratio. A trinitromethyl group would make it much more sensitive (and lead to thermal stability problems), because the NH group would no
longer be able to be electron-donating to the nitro groups.
Synthesis of 5-(Trinitromethyl) tetrazole from trinitroacetonitrile (II) and trimethylsilyl azide. The 5-(Trinitromethyl) tetrazole was isolated in
the form of its tetrabutylammonium salt.
"New Synthesis of 5-(Trinitromethyl)tetrazole", A. Shastin, T. Godovikova, B. Korsunskii
[Edited on 18-3-2012 by AndersHoveland]
|
|
|
Francis0126
Harmless

Posts: 4
Registered: 16-8-2011
Location: China Nanjing
Member Is Offline
Mood: It is hard to say ~~
|
|
Quote: Originally posted by AndersHoveland  |
I am not sure, but I think this would just form trinitronitromethyltetrazole, with the 3 nitro groups on the same carbon atom. Further nitration of
Fox-7, for example, is certainly possible, but much more difficult. Dinitromethyltetrazole would be even more resistant to nitration. It would
probably require N2O5 in HNO3. But I do not understand why one would nitrate it. Dinitromethyltetrazole probably already has a good sensititivity to
power ratio. A trinitromethyl group would make it much more sensitive (and lead to thermal stability problems), because the NH group would no
longer be able to be electron-donating to the nitro groups.
Synthesis of 5-(Trinitromethyl) tetrazole from trinitroacetonitrile (II) and trimethylsilyl azide. The 5-(Trinitromethyl) tetrazole was isolated in
the form of its tetrabutylammonium salt.
"New Synthesis of 5-(Trinitromethyl)tetrazole", A. Shastin, T. Godovikova, B. Korsunskii
[Edited on 18-3-2012 by AndersHoveland] |
Yeah ,I agree with you, the senstivity and thermal behavor are the questions for the -N-NO2 on the tetrazole ring.
I am here now,energetic materials!
|
|
|
| Pages:
1
2
3 |