| Pages:
1
2
3 |
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Off topic- Observation
The forum reminds me of a large table setting with many types of food....one takes the one to be enjoyed and the rest are for others which seek the
same joy with other foods from the same table......, hence take that which you like and leave the rest alone as it's not in your plate to either
comment or criticise........solo
[Edited on 7-4-2007 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tupence_hapeny
styrene monomer is widely available – albeit in an impure form |
Just anti-oxidants in small amount, distillation takes care of them
| Quote: | | although I am interested to know the role of the ‘blue-light’ in the sequence (as no mention is made in any of the patents of such)?
|
Unfiltered mercury arc is best, but harder for amateur to come by. Pumps more energy into the PS, aiding in bond breaking | Quote: |
As to the reactions, the epoxidation requires oxygen and an inert gas, would carbon dioxide suffice (as this would already be in the mix and moreover
is given off by the reactions). That would mean that oxygen could be added at a predetermined rate (based on the flow from the styrene
depolymerization reactor) along with some steam in the final step. |
Except on a fairly large scale the flow is not smooth and predictable
| Quote: |
Alternatively, bearing in mind that the object is to provide a workable synthesis, could a minor amount of oxygen be added to the carbon dioxide
during the styrene depolymerisation? This would cut down on unwanted side-reactions, while providing the oxygen for the next step – thus needing
only additional heat in order to complete the epoxidation step. |
How does O2 cut down on unwanted side-reactions in the depolymerisation? Adding a di-radical to a free raadical process you are.
| Quote: |
The phenylacetaldehyde coming from the reaction tube is of course going to be accompanied by unreacted styrene, three xylenes, and assorted dimers and
trimers (and tetramers), although (and providing the reactions work as advertised) these should be mainly styrene. |
The depoly step gives styrene, ethylbenzene, toluene, styrene oligomers, and traces of xylenes. The oligomers have a reactive double bond and will
react in the oxidation stage, as well as condense out if it's cool enough, and react with each other and the styrene to slowly plastic/gorp coat
everything.
| Quote: |
(2) reaction between phenylacetic acid and acetone;
(NB these patents actually claims to be useful in making BMK) |
That's right, BMK, standard lab method, not your target.
| Quote: |
(3) reaction between phenylacetaldehyde and acetic acid
|
Check the term Perkin's reaction, which is the common type for aldehyde + acid. The high reactivity of the benzl hydrogens may be enough to run the
way the patents say, I have doubts on that. It's a post Reagan era US patent, many of which are the equivalent of vapourware. Try it and see.
| Quote: |
Given that phenylacetaldehyde reacts with acetic acid to give the desired ketone (the patent is for cyclopropylketone) and phenylacetic acid reacts
with acetone to give the desired ketone, wouldn’t a fourth variant be at least theoretically possible? Being the reaction between phenylacetaldehyde
and acetone? |
PhCH2CHO + CH3COCH3 => PhCH2CH=CHCOCH3
And I'm too sleepy to type more.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tupence_hapeny
Finally, I have noticed numerous threads on this topic, the conversion of the polymer to the monomer, then to the aldehyde, etc. For the most part
such threads are silent on detail and specifics |
Why would someone bother depolymerizing polystyrene when he could just buy the damn thing for so little money? It might have been mentioned several
times, but only as a possibility or curiosity since polystyrene is a rare case where depolymerisation is actually possible. You can't expect anybody
trying out such a procedure just for the fun of it. You seem to find it more interesting than anybody up to now, so you try it out.
| Quote: | | As stated above, I am trying to dodge the Wilgerodt-Kindler style reaction, due to the level of byproducts which must be dealt with (although, and as
a recurring theme, could NMP [as a high-boiling amine] be used instead of pyridine or morpholine?). |
I wander why I even try explaining you anything when it is so damn obvious you do not read the replies to your post unless they contain some
ideological discourse. NMP is not an amine, it is an amide, something completely different and I already explained you in your other thread that it is
not a base! Obviously pyridine can not be used in the Willgerodt reaction as it is neither a primary or secondary amine nor ammonia, so like usually
you confused stuff. And again you demonstrate you read nothing of what is provided to you or you would have known that, by the Chinese patent in the
linked thread, practically no H2S is evolved unless in the hands of a fool.
| Quote: | | As to the reactions, the epoxidation requires oxygen and an inert gas, would carbon dioxide suffice (as this would already be in the mix and moreover
is given off by the reactions). |
Dry air is a mixture of O2 and an inert gas. Anyway, why would CO2 be amongst the reaction products?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
yes surf board shapers should and can buy pure monomer, its cheap , but
in mad max beyond thunderdome 2045 when the dude in the leather flight helmet finds a styrofoam cup he smiles knowing that each benzene ring will
help him fight off the evil oil bikers.....
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
Not_important,
I address your points, however, I will attempt to do so in a logical order.
(1) The pyrolysis of styrene (as is the case according to the attached article, with most polymers) can be carried out in a controlled manner by
controlling the temperature of the reaction vessel. In fact, the basic assumption of the author is that by conducting the degradation at the lowest
temperature at which it occurs, it is possible to keep the degradation to around 1%/min. This should be able to be worked upon in order to allow such
pyrolysis to take place at a steady, predetermined rate (I think the lowest temperature would be around 300C – perhaps less with a partial vacuum).
Alternatively, it may be possible to melt the styrene in an adjacent vessel, then deliver it to the reactor in a molten form (I suggest at or around
250C) at a predetermined rate. With vacuum it should not be necessary to heat it much further in order to commence depolymerisation, and as the rate
appears to be somewhat temperature dependent, it should be possible to establish some sort of constant rate (perhaps temperature * X = monomer /
minute or somesuch function, although I doubt it will be so simple).
Granted this stage of the reaction is purely speculative at present, however, this could be quite manageable. I would also suggest that instead of
merely purging the vessel and operating under a static blanket of carbon dioxide, a flow (in the appropriate ratio, as required by the epoxidation
step) could be delivered, with the additional benefit of drawing the liberated gaseous styrene monomer into the next stage of the reactor
(2) I appreciate the fact that many patents are rather more speculative than they appear, however, they should normally work to some extent. By the
way, the reaction between phenylacetic acid and acetone is claimed to result in Phenyl-2-propanone (NOT BMK, my mistake), with the acetone being
regarded as a precursor to acetic acid.
(3) I merely suggested the use of some oxygen (around 5% as opposed to pure air) as a compromise between pure air and carbon dioxide in the first
step. Even I am aware that oxygen will not prevent oxidation, however, surely the 95% carbon dioxide will prevent some of the oxidation experienced in
pure air. The 5% oxygen could then be used in the epoxidation step. I personally would prefer that a predetermined amount of oxygen (based upon the
rate of depolymerisation and in the proper ratio vis-à-vis the epoxidation step, was delivered directly prior to the epoxidation step.
(4) As to the condensation of the miscellaneous polymeric by-products, I would suggest that a trap is incorporated just after the product of the
depolymerisation step leaves the reactor. The product gas mixture should be allowed to cool to just above the boiling point of styrene (~100C I
believe), and by this mechanism the high-boiling compounds would not make it into the epoxidation step, having condensed and been collected in the
trap.
(5) The by-products remaining would be essentially the xylene, toluene and ethylbenzene (according to numerous papers, ethylbenzene is predominant at
some temperatures). These would presumably be oxidized during the epoxidation step (although I am unaware if they would form their respective acids or
aldehydes). The major remaining ingredient of this product would be the unreacted styrene, which could of course be recycled as a solvent for the
styrene prior the melting of the mix.
(6) I have not yet put a great deal of though into the separation step, however, this presumably would be possible, although fractional distillation
will be necessary.
Nicodem,
I have no response the first parts of the first parts (however, I am almost certain I have read someone claiming to have had success with pyridine
(here or elsewhere), obviously they did not).
So, the invitation from yourself to the person suggesting using the information in that patent, to feel free to poison themselves with the H2S was a
lie?
As to the last, CO2 is given off by over-oxidation in the epoxidation step. In fact, in the patent one of the x-methyl-styrenes gave essentially a
quantitive yield of the stuff
Roamingnome,
Thanks for that, I used to work with glass and I know the smell (and the bloody itch as well), however, all I had been able to find in normal glass
resins was 30-60% with polyester. I hadn't thought to search the specialist surfboard shops, they have got it. My thought process for this thread was
that if it were necessary for people to distill from a shitload of polyester - they might as well work in the gas phase - not valid.
[Edited on 8-4-2007 by tupence_hapeny]
Attachment: Luderwald, Ingo 'Mass Spectroscopy of Synthetic Polymers' (1982) 54(2) Pure & Applied Chem 255 (IUPAC).pdf (594kB)
This file has been downloaded 1367 times
We are all the sum of our experiences, and our reactions to the same
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
Sorry (re. double post) but as it is possible to purchase pure styrene monomer (again, my bad) the easiest method of getting to the phenylacetaldehyde
would be to epoxidise the styrene (such as the non-metal, hypochlorite option):
http://www.erowid.org/archive/rhodium/chemistry/epoxidation....
Distilling off the product, and passing the styrene oxide through silica gel (packed column style) a small amount of steam would be a good thing, as
would CO2.
The purified phenylacetaldehyde could then be oxidised further with Nickel Oxide Hydroxide which is (according to the article cited on this site) a
great agent for the oxidation of aldehydes to acids. It is used as a catalyst with hypochlorite.
Then choose whatever method you wish to get to the ketone.
[Edited on 8-4-2007 by tupence_hapeny]
Attachment: Non-metal.alkene.epoxidation (Styrene Oxide).pdf (63kB)
This file has been downloaded 1716 times
We are all the sum of our experiences, and our reactions to the same
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
chemsitry for the sake of chemsitry
since your on a 3 point shooting spree with this topic and the full paper on hypochlorite in butanol with Br seems appealing.
for your stated purposes it seems you should reach for the half-court shot with that epoxide substrate
for example
Abstract
The direction of the oxirane ring opening in the polymerization of styrene oxide by sodium methoxide was determined on the basis of the structure of
the products obtained. The oxirane ring opening was found to occur almost exclusively in -beta position. This result was compared with a model
reaction, i.e. the methanol addition to styrene oxide in the presence of sodium methoxide. In the model reaction the oxirane ring
opening occurred to ca. 35% in -position and to ca. 65% in beta-position.
The anionic polymerization of styrene oxide. Polymer structure and direction of ring opening
Die Makromolekulare Chemie
Volume 183, Issue 3 , Pages 587 - 591
i dont know what this means exactly and this paper probably bounces of the rim, but if you stumble across the "perfect reaction" be sure to post
it....
i looked into methyl radical addition. Not trying to sound sissy when i see all these sticky nasty steps happening, but ultimitaly the "elegant"
protocol is still yet to be unearthed. i.e if you dont need the PAA for purfume then this aldehyde creating and then making the acid form then ketene
lamping your self to p2p seems like an epic....
http://www.mdpi.net/ecsoc/ecsoc-/Papers/e0038/e0038.htm
pesky side reactions
[Edited on 7-4-2007 by roamingnome]
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
For those that think the science in this thread is lacking in something, perhaps this will alleviate your concerns somewhat:
http://www.niscair.res.in/ScienceCommunication/ResearchJourn...
So what you say, it is hardly likely that most of the people on this board, let alone a lowly drug-cook could make tetraphenylporphyrins? Well, I
would normally agree, however, I found this:
Gas-phase synthesis of 5,10,15,20-tetraphenylporphyrin:
http://greenchem.uoregon.edu/Pages/Overview.php?WhereFrom=Re...
MW Synthesis of 5,10,15,20-tetraphenylporphyrin:
http://greenchem.uoregon.edu/Pages/Overview.php?WhereFrom=Re...
Metallation of 5,10,15,20-tetraphenylporphyrin:
http://greenchem.uoregon.edu/Pages/Overview.php?WhereFrom=Re...
All we need now is for one of the 'actual' chemists on this board to say whether or not the procedure for metallating the porphyrin with zinc is valid
for metallating said porphyrin with Manganese. We would also of course require some details as to whether the 'acetate' of the porphyrin complex can
be formed directly or whether an involved procedure is necessary.
NB The 'lab procedures' link on the results pages gives a PDF copy of the actual lab procedure to make the porphyrin, which is for undergraduate
students, and not all that hard to follow.
tup
We are all the sum of our experiences, and our reactions to the same
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tupence_hapeny
Nicodem,
I have no response the first parts of the first parts (however, I am almost certain I have read someone claiming to have had success with pyridine
(here or elsewhere), obviously they did not). |
Well, now that I'm more familiar with how your confused mind works I can claim that you confused pyridine with piperidine.
Am I right?
| Quote: | | So, the invitation from yourself to the person suggesting using the information in that patent, to feel free to poison themselves with the H2S was a
lie? |
Fools are always in danger of poisoning themselves when playing with chemicals, that's why the warning. Does a cook know what the hell is he doing?
Does he know he must not acidify the polysulfide solution? No he does not - that's why the warning.
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
| Quote: | Originally posted by Nicodem
| Quote: | Originally posted by tupence_hapeny
Nicodem,
I have no response the first parts of the first parts (however, I am almost certain I have read someone claiming to have had success with pyridine
(here or elsewhere), obviously they did not). |
Well, now that I'm more familiar with how your confused mind works I can claim that you confused pyridine with piperidine.
Am I right?
| Quote: | | So, the invitation from yourself to the person suggesting using the information in that patent, to feel free to poison themselves with the H2S was a
lie? |
Fools are always in danger of poisoning themselves when playing with chemicals, that's why the warning. Does a cook know what the hell is he doing?
Does he know he must not acidify the polysulfide solution? No he does not - that's why the warning. |
Nicodem,
mmmmmm, you may be right in regard to the first part........
As to the second, I don't believe that it is possible to download the relevant patent's translation from that topic.
Next, what no comment on the porphyrins?
Maybe I should find a topic where they may be discussed?
We are all the sum of our experiences, and our reactions to the same
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tupence_hapeny
...
Next, what no comment on the porphyrins?
Maybe I should find a topic where they may be discussed? |
Topic drift, while not a mortal sin, can be a venial one.
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
not_important,
Yet how is it topic drift, considering that TPP is the only agent yet discovered that can transform styrene to its oxide in 100% yields? I find a
step-by-step synthesis of TMP that is so easy even k3wls could do it & you call that topic drift?
All I need now is a synthetic route for iodosylbenzene...
tup
We are all the sum of our experiences, and our reactions to the same
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Your tendency to complicate and confuse is admirable, but yet of little practical value.
There are hundreds of methods for the preparation of styrene oxide published and most are more practical than any you proposed up to now. Why would
anybody use some weird unpractical method when the same thing can be performed by a simple method using either TCCA, Oxone, H2O2, sodium perborate,
sodium percarbonate or even common bleach, all chemicals that can be obtained simply by going shopping to the nearest consumerism worshiping temple. I
thought you were after a simple, fully OTC method? Do you really expect any of us seriously discussing about methods that have no use beside the
academic interest or potential industrial application? Get grounded to the earth and perhaps there can be found a topic worth discussion. This is a
forum for amateur science.
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
Look, I find it extremely exciting that amateur chemists can now synthesize such advanced structures, the use of which is yet to be fully explored by
the scientific community. I admit porphyrins have little utility in this OTC type thread, however, I still believe that they are of some interest.
That being said, and the fact that the hypochlorite/bromine epoxidation with the silica isomerisation followed by nickel peroxide to the acid,
basically provides a viable OTC route to phenylacetic acid - I have started a thread on the porphyrins and will now leave this thread to whomever
finds it of utility.
Is that okay with you?
tup
We are all the sum of our experiences, and our reactions to the same
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
i hope you dont mind me dropping this here, it is somewhat unrelated to a small minority however you could repeat the kornblum oxidation to obtain p2p
instead of the last step using NH3 resulting in the censored product
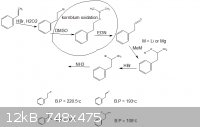
[Edited on 14-7-2012 by rannyfash]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Electrosynthesis of phenyl-2-propanone derivatives from benzyl bromides and acetic anhydride in an unsupported micro-flow cell electrolysis
process
Ping He , Paul Watts , Frank Marken and Stephen J. Haswell
Green Chem.
2007,9, 20-22
DOI: 10.1039/B610415K
Attachment: Electrosynthesis of phenyl-2-propanone derivatives from benzyl bromides and acetic anhydride in an unsupported micro-flo (243kB)
This file has been downloaded 2370 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
polymerizer87
Harmless

Posts: 35
Registered: 16-7-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | i hope you dont mind me dropping this here, it is somewhat unrelated to a small minority however you could repeat the kornblum oxidation to obtain p2p
instead of the last step using NH3 resulting in the censored product
[Edited on 14-7-2012 by rannyfash] |
I'd like the source to this, interesting.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
sounds like a stinky sulfur run too me and
a heap of stress about lead posioning XD
otherwise superbase DMSO and off to desired target.
e3500 console login: root
bash-2.05#
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
this is the source, its oc
|
|
|
polymerizer87
Harmless

Posts: 35
Registered: 16-7-2012
Member Is Offline
Mood: No Mood
|
|
Does anyone have synthetic experience with the Willgerodt Reaction with Styrene to phenylacetic acid? I'm more curious about the odor it puts out...
Morpholine and styrene have a very familiar smell to them, and let's not forget sulfur. I won't attempt this reaction if it's going to bring attention
to my home. I don't have any desire to carry this precursor to p2p. I just want to use up the 4 liters of morpholine and styrene that I have.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Not quite useful for what you guys are talking about, but did you know that direct air oxidation of molten polystyrene will yield a useful amount of
benzaldehyde? Keep the temp between about 280-330 in order to minimize the styrene monomer coming over with it. It won't yield a pure product out of
the condenser. Using bisulfite adduct would get you nearly clean benzaldhyde though. It is by far the major oxygen containing product other than CO,
CO2, and H2O. The only other things that could get caught as a bisulfite adduct are minute traces of cinnamaldehyde and acetaldehyde.
In this paper where they tested the decomposition properties of 'polycubes' (PuO2 suspended in polystyrene) it was able to produce a 28% by weight
yield of benzaldehyde, plus 14% styrene, 4% toluene 2% benzene, 0.3% indane, everything else organic under 0.1%. And that was with highly crosslinked
PS containing CeO2 as stand in for PuO2. They go on to say that crosslinking and CeO2 result in lower levels of flammable products, more char. If you
used normal PS and actually optimized for benzaldehyde >40% should be possible, but no one has ever tried AFAIK. Also it has the added advantage of
being a faster and lower temperature process vs inert gas pyrolysis.
http://www.osti.gov/bridge/servlets/purl/808247-zEzoGI/nativ...
I realize you might get a higher yield by a inert gas pyrolysis (or trip to the paint store) then liquid phase oxidation of styrene monomer. But that
a whole second step with reagents and all that... Polystyrene and heat are just so much more cheap and available, this can't be ignored. I have no
doubt people somewhere in the world would appreciate this.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
yes, i was reading about strait destructive pyrolysis to crude benzaldehyde some where else. From what i gathered the styrene can be self polymerized
with oxygen, and this intangels oxygen in the polymer.
When it is thermally decompositioned the aldehyde can be significant.
A metal oxide catalyst may direct the reaction products even more.
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
just another quick thought, ozonolysis of liquid styrene produced from the dry distillation of polystyrene, benzaldehyde and formaldehyde
[Edited on 13-8-2014 by rannyfash]
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
Entirely OTC-ish:
Benzaldehyde, Ethyl acetate, NaOH, NaBH4, H2SO4:
Benzaldehyde + Ethyl acetate + NaOH (tiny amount, e1cb) --> diketone
diketone + NaBH4 --> glycol
glycol + H2SO4 --> P2P (https://www.erowid.org/archive/rhodium/chemistry/peracid.htm...)
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
You might have already known that NaOH does not dissolves in ethyl acetate, neither it does in benzaldehyde, and it reacts irreversibly with ethyl
acetate in presence of water, and gives Cannizzaro reaction with benzaldehyde.
|
|
|
| Pages:
1
2
3 |