| Pages:
1
2 |
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Xiomy; if you are doing this in a university surely there is someone you can ask and presumably you have library access to online journals? If not
will your university have N,N-bis(2-pyridylmethyl)amine? This is the most difficult part of the preparation. I am interested in this ligand for other
purposes and am in the process of trying to make. I have got as far as 2cyanopyridine; from here I propose to try and reduce it to the mono-amine
2-pyridylmethylamine then condense this with 2-pyridinecarboxaldehyde to produce the enamine. Then I'll have to find away to reduce the enamine to the
desired product.
Then you have to carry out the syntheses above. No mean feat; I managed to acquire some 2-picolinamide and 2-pyridinecarboxaldehyde which helped a lot
but the dehydration of the amide was a tough nut with poor yields and the selective hydrogenations that are then required have brought my experiments
to a halt for now.
So good luck and keep us posted on your progress!
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
2-methyl pyridine is also known by picoline; it is a industrial bypriduct.
Boffis, why didn't you skip the nitrile, unless you started without the aldehyde and then bought that? Reduction might be tricky because the pyridine
nitrogen will want electrons, which I suppose you know, but I think you can manage.
I would have thought 2-halomethyl pyridine (eg. 2-Picolyl chloride) to be a superior starting point, though: because of its bulk you could probably
only stir it together with ammonia and get a good amount of dialkylated product.
I have found you a reference to its preparation from picoline, if that interests you, via chlorination with trichloroisocyanuric acid,. http://onlinelibrary.wiley.com/doi/10.1002/cber.19871200431/...
[Edited on 21-1-2018 by halogen]
[Edited on 21-1-2018 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@halogen; I didn't have any picoline at the time I started though I was keeping an eye open for it and still am but I did track down some picolinamide
which is rare, the 3 carboxamide is nicotinamide but is no use in this case. Another problem with the chloromethyl group to amine is getting the right
amine! You can get the mono amine by using the Delepine or the K-phthalamide reactions but getting the the secondary amine is more difficult, you tend
to get mixture which then has to be separated. The advantage of the enamine route was that it only gives a secondary amine (in theory). So while you
are right about pyridylmethyl chloride being a simpler route to the monoamine, the secondary amine I am less sure about and I also have other uses for
pyridine-2-carboxaldehyde (I have already posted the preparation of pyridoin and I am now trying to convert it to 2-pyridil (the pyridine analogue of
benzil)) so it made sense to purchase some when the opportunity arose.
It may be possible to separate the mixture of pyridylmethyl amines that results from the interaction of ammonia and the pyridylmethyl chloride in a
manner similar to that used to separate aniline, methylaniline and dimethylaniline but that's more research and experimentation.
As with most amateurs the route I choose is to a large extent dictated by what is available to me! If only we could still pick up the Sigma A or TCI
catalogue and just buy what we need ... Ahhhh Dreams 
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
An interesting material however i dont think equipment is the issue.
http://pubs.rsc.org/en/content/articlepdf/2014/sc/c4sc01636j...
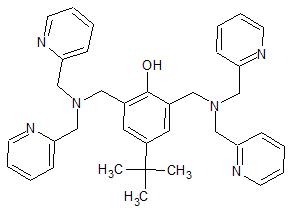
This i think it bpbpH, to which i estimated the structure based on the following paper:
https://www.sciencedirect.com/science/article/pii/S002016930...
It is this compound that i think is the troublesome part.
Co(BF4)2.6H2O i think could be prepared by reacting hydrofluoric acid with boric acid to form fluoroboric acid, which could then be neutralized with
cobalt hydroxide and then worked up.
I originally tried to draw the entire {(bpbp)Co2II(NO3)}2(NH2bdc)2.2H2O ligand complex on chemsketch however it turned into a bit of an illogical
mess and while it does resemble the compound from the paper, i think the structures in the paper do a much better job of illustrating it  . .
[Edited on 23-1-2018 by Assured Fish]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
In general yes: in the case with benzyl chloride very little primary amine is produced; the secondary and tertiary are separated by crystalization as
hydrochloride and or distillation the aryl groups are less trivial than methyl (and the three basic amines is leverage w/ pyr) (interestingly
tribenzylamine when subject to notably rough treatment but relatively simple... heated to 250 deg. in a stream of HCl yields Bn2NH salt, that wouldn't
work with pyridyl, just a fun fact); because the pyridyl nucleus is more miserly with its electrons, by comparison to benzene, I supposed the
di-aralkylated product to be reasonably expected in good yield but I could be wrong
https://books.google.com/books?id=XIYPAQAAIAAJ&pg=PA337
https://books.google.com/books?id=dqs0AQAAMAAJ&pg=PA2068
https://books.google.com/books?id=UngPAQAAIAAJ&pg=PA491 (for fun)
In any case, good luck.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You can probably make bis-(2-pyridylmethyl)amine by the reaction of p-toluenesulfonamide with two equivalents of picolinyl chloride
(2-chloromethylpyridine) in the presence of a suitable base, followed by deprotection with HBr in AcOH. This then condenses with formaldehyde and
4-tertbutylphenol to the ligand. This detosylation is disclosed here:
http://pubs.acs.org/doi/abs/10.1021/jo00863a042
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@clearly_not_atara; I looked at the link above and I have to say I don't see any support for your idea! However, pursuing this logic acid; amides are
usually fairly uncooperative with regards to substitution into the NH2 group what make toluene sulphonamide special? If this work would say benzamide
or acetamide work? I once required some pure N-methylaniline and I wondered if you could prepare it from acetanilide by deprotonating the remaining
amide hydrogen and then reacting it with methyl iodide. I did find a little about this reaction but it is evidently very obscure but displacing both
protons from a sulphonamide group! It would be a nice route to to secondary amines if it worked.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The link is only supposed to describe deprotection, not alkylation. I thought it well known that when amides are deprotonated, they are easily
alkylated. Sulfonamides are about 1000000 times as acidic as carboxamides; P-toluenesulfonamide has a pKa of 10.2 whereas that of acetamide is 16.5.
Deprotecting sulfonamides is usually the "hard" part of using N-sulfonyl protecting groups so in the citation the "superacid" system of HBr in AcOH
does the honors, and this is preferred to alternatives like Birch reduction (which will destroy pyridine) or trimethylsilyl iodide (which is not OTC).
A description of the preparation of secondary amines via the base-catalyzed alkylation of sulfonamides can be found in Hu et al 2004, attached. Note
that some of the problems Hu was concerned about when using strong bases in organic solvents (dehydrohalogenation) are absent here because
2-chloromethylpyridine does not undergo dehydrohalogenation.
Attachment: hu2004.pdf (69kB)
This file has been downloaded 414 times
[Edited on 24-1-2018 by clearly_not_atara]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Clearly_not_atara: I didn't know there was such a difference between carboxamides and sulphonamides. This is interesting since it opens up a whole new
route to secondary amines. I see now the significance of the paper you posted earlier.
Just have to find some picoline now
|
|
|
| Pages:
1
2 |