| Pages:
1
2
3 |
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
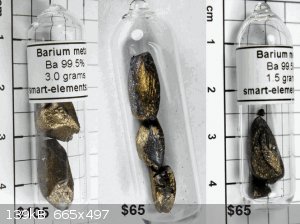
As a yardstick of progress, I compare my samples to the best larger ones that I can find, the ones at Smart-elements. Although, their largest was
(they are out of stock) 3 grams, while my smallest is about 6 g.
Comments on comparisons? Theirs are kind of pricey, like 30 - 40 dollars/g. I don't know why it couldn't be a third of that?
My first "brighter" looking samples were sunlit, the last incandescent, so bear that in mind. My first two pictures look wayy more silver than I
actually see them, but it does shows that they are quite reflective.
Their samples look almost tumbled, what do you think? Yes, I'm still kinda' beating that horse. I'm surprised a commercial enterprise doesn't have a
glovebox capable of better than this. Maybe they use M. Braun?
[Edited on 26-1-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am loving this Dan. I think your samples look superior. Larger, brighter and I prefer the cut look to the rounded edges -- even if tumbling could
potentially produce a brighter finish, they haven't achieved it.
For my element collection, Ba is a bit further down the list. But if you get your methodology perfected then I might reprioritise. Count me as a
potential customer!
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
It's been some time since I've given much thought to shiny Ba metal.
Interestingly, our company is currently designing a custom glovebox for a university in the SE. A requirement is to handle metallic Ba metal and keep
it clean. I’ve been involved in devising modifications to improve the normal glovebox atmosphere and so I've had reason to consider the root of the
issue in more detail.
The challenge is rooted in the actual mechanism of the discoloration. When I examined the best Ba sample that I've made, there were two simple
observations that seemed significant. The metal surface is a multicolored affair and it looks like a metallic surface, smooth and shiny, not dull. The
pattern of colors resembles oil on water. And, similar to the oil-water system, this must be caused by a variation in thickness of the coating. But
this must be on the same scale as the wavelength of light. The sad truth is that barium tends to react, when only trace impurities are around, to give
a highly refractive surface.
This is different than Cs, where impurities dissolve into the metal, or other alkali metals, which only lose a small amount of reflectivity when the
surface hosts trace impurities, and no additional colors are produced.
IMHO, barium is uniquely poised, along with strontium, to provide the most difficult challenge among all of the stable, naturally occurring elements
to successful manipulation without degrading the surface appearance.
Has anyone ever seen a silvery sample of barium metal in an ampoule? I can't even find a picture of it.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Just a thought.
The 2 samples above look from their textures cast from molten state or electro refined/CVD (if that's even possible), suggesting they have not been in
contact with other metals or their oxides/hydroxides in passivation layers.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Interesting stuff. Not read this whole thread before now.
So the main challenge is to manipulate the Ba without letting anything get near it, like in a perfect vacuum ?
Got an idea lying about in the back of my mind (such as it is).
Something to do with a cast aluminium reactor vessel that can be sealed, heated and vacuumed, with actuators inside to move things around.
Lose the transparent materials, also the hand-holes to make it simpler and easier to build.
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
Aren't barium animus what they give you at the hospital so your colon X-rays are easier to read?
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Hi j_sum1,
Those were made for Galliumsource a number of months ago.
Dan
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by froot  | Just a thought.
The 2 samples above look from their textures cast from molten state or electro refined/CVD (if that's even possible), suggesting they have not been in
contact with other metals or their oxides/hydroxides in passivation layers.
|
Hi Froot,
I've been looking for hints of their method in the shapes of the samples. Yes, they have that smooth look of something solidified from molten metal.
But still, they are far from acceptable samples. I wonder how Ba looks right after distillation by the producer? I mean, does the first fraction
effectively scavenge all impurities so that subsequent matl. is clean? I guess it doesn't matter, they have to collect it, it can't stay silver.
RGB in England is putting together a specially designed glovebox (at least a year ago) to make clean samples. They haven't delivered yet. They aren't
using hot Ti to scavenge N2 so I have some doubts if it's possible.
Dan
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by aga  | Interesting stuff. Not read this whole thread before now.
So the main challenge is to manipulate the Ba without letting anything get near it, like in a perfect vacuum ?
Got an idea lying about in the back of my mind (such as it is).
Something to do with a cast aluminium reactor vessel that can be sealed, heated and vacuumed, with actuators inside to move things around.
Lose the transparent materials, also the hand-holes to make it simpler and easier to build. |
Hi aga,
I know what you're thinking. It's also my fear that only a sophisticated purpose-built device may be enough.
Dan
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Hi Blogfast,
That bronzy (holy shit, that's a real word?) look is reminiscent. Strange little story, probably 15 or 20 years back, I prepared a drug for NCI. It
was so unusual that out of the thousands of molecules I made, it stands out. It was a disubstituted benzoquinone, that's the only details that I
recall, and it had no metal in it. But it was just like bronze powder..exactly. No hint of any other color. I didn't even know that was possible given
that gold isn't a color, but more a byproduct of relativistic quantum effects.
Dan
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I wasn't thinking 'sophisticated', just 'purpose-built' which could turn out as simple as a tennis-ball sized aluminium casting and a few magnets to
move the actuators from the outside, eliminating the need for any holes to do stuff.
Basically a box (probably spherical) with the minium size and abilities to do what you want and one hole to put the stuff inside and also to suck the
air out/introduce argon.
If it's Aluminium, easy to make, easy to heat to say 300 C to drive out any water without compromising the structure.
Lob in your material, suck out the air, let the 'getters' get whatever they're supposed to get from the very small volume, then process the material
as the design required using the ouside Nd magnets.
I dunno if that would work. Just an idea.
Also sounds like great fun to make !
If i can possibly make an apparatus that helps, let me know and i'll try to make it and send it if possible.
It would be awesome for an amateur effort to reliably make shiny ampouled elemental Barium.
The septum idea before sealing the ampoule sounds like a good do-able solution.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
And that, my friend, was the true inspiration for the tongue-in-cheek title.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Hi Dan
I followed your comments about the frustrating struggle to get shiny barium chunks.
What about heating in vacuo in order to dissolve the dark layer into the barium mass?
At 727°C it melts so it is not too high temperature for a small oven in quartz tube with a flange to a vacuum system.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by aga  | I wasn't thinking 'sophisticated', just 'purpose-built' which could turn out as simple as a tennis-ball sized aluminium casting and a few magnets to
move the actuators from the outside, eliminating the need for any holes to do stuff.
Basically a box (probably spherical) with the minium size and abilities to do what you want and one hole to put the stuff inside and also to suck the
air out/introduce argon.
If it's Aluminium, easy to make, easy to heat to say 300 C to drive out any water without compromising the structure.
Lob in your material, suck out the air, let the 'getters' get whatever they're supposed to get from the very small volume, then process the material
as the design required using the ouside Nd magnets.
I dunno if that would work. Just an idea.
Also sounds like great fun to make !
If i can possibly make an apparatus that helps, let me know and i'll try to make it and send it if possible.
It would be awesome for an amateur effort to reliably make shiny ampouled elemental Barium.
The septum idea before sealing the ampoule sounds like a good do-able solution. |
The idea is entirely logical and one that I've thought about.
I think the sophisticated part comes in when you have to be able to exert enough mechanical force to cut and shave the metal. So, then you come right
down to it...you either incorporate gloves (and just maybe that is enough to cause it to fail) or you make the mechanical parts capable of grabbing
crude chunks, cutting and cleaning and all of the manipulations required to do that.
My thought is to just distill the barium directly into a quartz ampoule. I need to do more research on that idea. I'll have the quartz and an
oxy-hydrogen torch from my current black P project.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by Heavy Walter  | Hi Dan
I followed your comments about the frustrating struggle to get shiny barium chunks.
What about heating in vacuo in order to dissolve the dark layer into the barium mass?
At 727°C it melts so it is not too high temperature for a small oven in quartz tube with a flange to a vacuum system.
|
Whoa!
Now there's an idea that has never occurred to me. It has the feature that I think is most important, simplicity. Every manipulation is just
another opportunity to fail.
Since this never crossed my mind, I don't know the fundamental answers about solubility and stability that need to be examined. But a Kugelrohr of
quartz that heats to 700-800 C is really attractive.
Whether for straight up distillation or dissolution of impurities into the bulk, this is an intriguing idea.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
God, you just have to wonder...is a two-stage vacuum pump clean enough? There is always a trace of oil vapor in the evacuated space. How much is too
much? Not an answer that is likely to be found without trying it, I think.
[Edited on 21-11-2016 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Try and try again, and enjoy !
Finding stuff out is wonderful fun.
Edit:
If a space is small, how about attaching a tube from it to an enclosed reaction that rips oxygen out of the atmoshere ?
Ditto any other unwanted contents of the reaction vessel.
A vacuum isn't the only way to rip stuff from the atmosphere, especially if that volume is small.
[Edited on 21-11-2016 by aga]
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
I am used to work with diffusion and turbo pumps.
Also I understand that they are not always at hand of all amateurs.
Probably a two-stage mechanical pump and a cold trap to stop oil backstreaming could be enough.
Other approach could be no vacuum at all. Only an inert atmosphere, with ultapure argon.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
It's obvious that only quartz will suffice here. Trying things over and over is one thing in borosilicate, entirely another in quartz.
Quartz can effectively be baked in vacuo to give a very, very clean surface. The Ba will already have oxide and nitride, so any residual O2 and N2
after pumping will only be scavenged during heating of the Ba.
I have UHP argon, but this presents a problem. The bp of Ba will be excessive.
The vacuum needs to be good enough to lower the boiling point of barium to a reasonable level, and it must be clean. Trapping is the obvious answer.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
so, are you able to run a test?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
I had always heard that the shiny stuff at the end of an old time vacuum tube was usually barium, evaporated as the "getter".
This provides an account of how that barium getter was created:
https://en.wikipedia.org/wiki/Getter
It used barium azide that was decomposed through induction heating, with the resultant nitrogen pumped out. They were using ordinary glass tubes for
this. Perhaps you could manage to coat the entire inside surface of an ampoule.
Due to the specifics of the described process, I suspect that any ordinary vacuum tube with a shiny getter really is barium.
About that which we cannot speak, we must remain silent.
-Wittgenstein
Some things can never be spoken
Some things cannot be pronounced
That word does not exist in any language
It will never be uttered by a human mouth
- The Talking Heads
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Not yet. I'm acquiring quartz tubing and an oxy-hydrogen torch presently, for a black P project.
Right now, this is just in the early exploratory stages.
Waiting until our client starts his Ba work in his new glovebox should give me some invaluable practical info.
Careysub,
Yes, that is almost always barium. I believe cesium was also used at one point, but barium is probably superior.
This passage is interesting:
Barium's industrial applications are limited because of difficulties in use. Usually barium is placed into a protective shell made
from another metal or used as an alloy. Sometimes barium is obtained directly in the apparatus it is supposed to be used in by reaction of barium
oxide with aluminum. Barium alloys with magnesium and aluminum are used in high vacuum apparatus as a residual gas absorbent (getter).
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Dan Vizine  |
I think the sophisticated part comes in when you have to be able to exert enough mechanical force to cut and shave the metal |
No idea how hard Barium metal is, but have an idea how Levers, reduction gears etc work to increase the Force applied to items.
If it's not as hard as steel, some small, magnetically operated machine should be able to shave it.
I do not know if Ba would react with the elements present in steel.
If it does, that idea would not work at all.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by aga  | Quote: Originally posted by Dan Vizine  |
I think the sophisticated part comes in when you have to be able to exert enough mechanical force to cut and shave the metal |
No idea how hard Barium metal is, but have an idea how Levers, reduction gears etc work to increase the Force applied to items.
If it's not as hard as steel, some small, magnetically operated machine should be able to shave it.
I do not know if Ba would react with the elements present in steel.
If it does, that idea would not work at all. |
It could probably be handled by clean, dry steel tools at room temperature. And, I imagine that any impurities adhering to the tools will end
up in the Ba. Ba, btw, is a bit harder than lead.
It's my present view that only simplicity can yield good results.
The only question in my mind is how, exactly, to do it.
I won't know how good I am at working quartz tubing until I actually do it.
The apparatus needs to be simple. You can do amazing things with a straight tube with constrictions. I process Na, K, Tl, and Pb that way. Pics of the
higher melting are shown, Pb first and Tl sitting on a magazine cover. It's interesting to see these metals as shiny & silvery.
The conditions are demanding:
Barium is a silver-white metal, not as soft as lead, and of density 3.78. It melts at about 850° C, is volatile at 950° C, and boils at 1150° C
in vacuo.
But, working at modest diameters (maybe 16 mm, certainly 10 mm) and with thick walls (2 mm), maybe I can work this out. This is the first time that I
feel I have a real shot at this. In fact, black P just became less interesting.
How much better it is to make a unique product!
I wonder if I can plunder the available literature on getters to any practical advantage?
 
[Edited on 22-11-2016 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Maroboduus
Hazard to Others
  
Posts: 257
Registered: 14-9-2016
Location: 26 Ancho Street
Member Is Offline
Mood: vacant
|
|
This is a fascinating thread.
Sounds like getting the surfaces clean without their re-tarnishing as you work is a big part of the problem.
What if you had a container of some liquid that doesn't react with barium in there and filed or ground the surfaces clean under the liquid? Then you
could quickly pull the sample out of the liquid, shake it off (hopefully a low viscosity liquid), or blow it dry with a jet of argon, and stick it
into the sample tube as quickly as possible?
Or stick it in the sample tube wet and blow it dry in the tube with a jet of argon, maybe with a cap on the tube which allows purging the tube with
fresh argon from the tank.
This is a total shot in the dark, so don't assume I actually know what I'm talking about.
|
|
|
| Pages:
1
2
3 |