| Pages:
1
2
3 |
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
IIRC xylene can be used to store sodium.
Edit: Do not know about petroleum ether but problem with many solvents is that oxygen and water are somewhat soluble in them. Not much is needed to
eat all your sodium away with month or two.
[Edited on 20-8-2006 by chromium]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I think it is not more commonly stored under lower boiling liquids because it could evaporate during storage. In the lab I worked at the majority of
air reactive metals(alkali, alkali earth, rare earth, actinides) were stored in bulk under oil and small ammounts were stored separatly under hexane.
|
|
|
samdar
Harmless

Posts: 6
Registered: 11-8-2006
Member Is Offline
Mood: No Mood
|
|
very danerious to store under ether because most ether and other solvents are not dry when you by them and may ignite with the sodium. Stick to the
mineral oil. You can drive the oxygen out of the oil by bubbling nitrogen through it.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
It can be kept under pet ether, it's just a mixture of fractions. As a matter of fact, one of the bottles in the lab has bottled sodium sitting under
pet ether.
I like neutrino's method of just melting it, it's not hard to do with a warm oil bath and not particularly dangerous. Still, it would be incovenient
for anything but long term storage.
Anyone thought of vacuum packing it?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
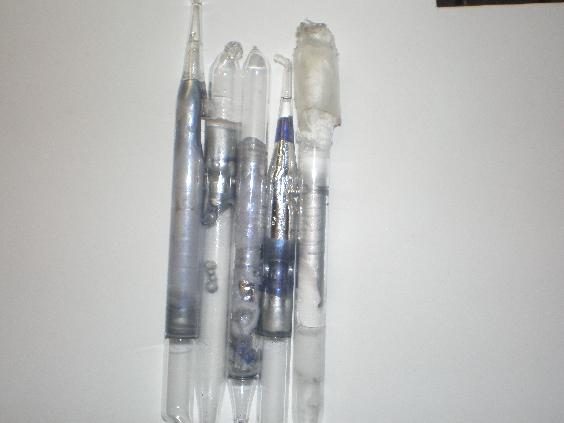
I figured I would make a few little sodium ampoules today. I melted sodium under parafin oil/mineral oil and sucked it up in a pipet which I then
sealed with a torch. Some turned out nicer than others. Melting was simply done over an alcohol burner, and the shinier samples I think are a result
of the temp of the molten Na.

|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I have some sodium bricks I was thinking in wraping in fiberglass cloth then dipping in melted microcrystaline wax for long term storage, sort of like
storing cheese. So far, they seem to be holding up just fine wet with heavy mineral oil and double ziplock baged with all the air pressed out (3
years or so).
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
As long as the bag is sealed shut perfectly (and we discount diffusion through the plastic  ) with say melting, the sodium will remove all of the CO2 and O2 out of the atmosphere of the bag. I store cut pieces of sodium under
mineral oil, but that's only because they have a higher surface area and I want to keep them clean. The larger logs I keep in a large bag that I've
melted shut, then put in another bag and melted shut again. The sodium creates a partial vacuum in the first bag because it reacts to form NaOH,
Na2CO3, and Na2O. The oxidized portion is a very thin crust (maybe less than a fraction of a milimeter). ) with say melting, the sodium will remove all of the CO2 and O2 out of the atmosphere of the bag. I store cut pieces of sodium under
mineral oil, but that's only because they have a higher surface area and I want to keep them clean. The larger logs I keep in a large bag that I've
melted shut, then put in another bag and melted shut again. The sodium creates a partial vacuum in the first bag because it reacts to form NaOH,
Na2CO3, and Na2O. The oxidized portion is a very thin crust (maybe less than a fraction of a milimeter).
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
labi
Harmless

Posts: 15
Registered: 12-10-2005
Member Is Offline
Mood: No Mood
|
|
youcan best store it in kerosene or liquid paraffin that is how i was storing about 100gm in my lab from a year
|
|
|
prole
Hazard to Self
 
Posts: 94
Registered: 4-8-2005
Member Is Offline
Mood: No Mood
|
|
Hello all,
I've suddenly found myself in possession of a little sodium. I hastily threw a storage system together because the block was covered with oxides and
I didn't want raw sodium sitting around. I tried a small chunk in mineral oil, and immediately hundreds of tiny bubbles, presumably hydrogen,
evolved. I then threw another small chunk in some kerosene, and got the same bubbles. No fizzing, hissing, popping, exploding... Just a gentle,
smooth bubbling, which subsided within about 20 minutes. I put the main chunks in a metal paint can, and covered all with kerosene to the top of the
can, to limit air exposure. I went with kerosene because I feel that it will be easier to rinse off than mineral oil. Now, with the bubbles, I'm
slightly concerned about pressure building up in the can and blowing the top off. Should I be worried? I'm gonna burp the can later to see if any
pressure built up. Is the can, which is lined with some corrosion resistant material, a safe place to store sodium? I was gonna use a mason jar, but
the chunk wouldn't fit through the hole, and I didn't want to cut it all up. I'm also thinking about sealing the lid with rubber cement, or similar
to further keep out air and moisture. I feel it's safe at the moment, but any input from y'all would be greatly appreciated. I'm after SAFETY FIRST
and then preserving as much of the beautiful metal as possible.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Mineral oil contains measurable water unless it has been dehydrated. You've dehydrated yours.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Here is a picture of that sodium metal from the can mentioned up above for reference. This was rather dirty sodium, but at least you all can see how
soft it is. I am cutting it up on aluminum foil, like an idiot, but it was a spur of the moment thing. I could actually hear the hydroxide hissing and
popping as it reacted with the aluminum. This was for reaction with anhydrous methanol to produce Na methoxide. The scrap trimmings went into a
stockpot and were disposed of  Apologies for the large photo. Apologies for the large photo.
[Edited on 2-1-2007 by Fleaker]

Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I`ve got mine stored in a food jar (airtight) under pharmacutical grade white parafin, it`s been there for over 5 years and beyond a little surface
tarnish, it`s still perfectly fine and viable.
The Davster, I really like what you`ve done there! I stofe most of my elements in such ampuoles (also home made), I just havent gotten around to doing
the Na yet, thnx for the Idea/Method 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
I store my sodium in a tightly sealed bottle under parafin oil.
Holds up just fine!
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
OK weird...remember Jdurg's red potassium? Well my homemade sodium ampoules turned blue. . Only the shiniest sample did not turn blue. . Only the shiniest sample did not turn blue.
(pics tomorrow or day after, camera borrowed)
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by The_Davster
OK weird...remember Jdurg's red potassium? Well my homemade sodium ampoules turned blue. . Only the shiniest sample did not turn blue. . Only the shiniest sample did not turn blue.
(pics tomorrow or day after, camera borrowed) |
Wow! I'm pretty sure that my potassium is an ozonide compound, but I'm still not sure about that. This blue color on the potassium also takes me by
surprise. I wonder what the hell is going on here to create these odd colored ionic compounds, and what they heck they are. (As an update to my
potassium, there is now a huge covering of metallic-red ionic substance over the surface of it. Yet, in other areas parts that used to be covered by
a white oxide are now completely metallic and oxide free. I really wish I could donate this sample to a research lab and find out what the heck is
going on.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
A similar thing I noticed with cerium, stored under mineral oil. First, the sample was shiny, like this (I scratched away some of the blue layer):

Lateron it turns blue again:

So, this effect seems to be quite common and there are multiple metals, which are affected by it.
[Edited on 14-1-07 by woelen]
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Nice sample woelen. I know that cerium forms a green oxide compound when stored over time in a non-inert atmosphere. I would think that blue
coloring would be possible as well due to the similarity between green and blue. (One little change in the wavelength and you get green as opposed to
blue. Layer interference could cause that as well). It will be interesting to see the blue color on his sodium sample though.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Here is the pic, some of the blue washed out though. It is important to note that blue 'stuff' formed on the areas of sodium where upon making the
ampoules there was only a very thin layer of oxide on the sodium. Areas where there was absolutly no oxide layer were unaffected, and an amopule
where an end was broken off allowing oxygen slowly in, did not show a blue color as it oxidized. The 3 leftmost are lighter blue than the second from
the right, some colour washed out in the picture. Far right is the one open to the air, and has no blue whatsoever.
So far, what seems to happen is that the blue stuff forms on a thin oxide layer between sodium metal and glass when no more oxygen is allowed to get
to it. Leading me to think it is some sort of suboxide. It could be some sort of reaction with glass as well, but Jdurg's sample is not in contact
with glass. Could also be some sort of solid solution of sodium in sodium oxide that reflects light differently.
I have had the opportunity to work with the first 4 lanthanides by the kilo (they made the summer student(me) hacksaw the pieces into smaller
pieces ), so I saw all of them in their various oxidized states. When lumps of
each are only exposed to the air for a few hours, usually a deep blue oxide layer forms on La, Ce, and to a lesser extent, Pr and Nd, Pr and Nd are
still rather shiny after such time. After a few days on small pieces of the metal, they are simply a white powder. Never expierenced this with the
ingots,these were too precious to leave out. ), so I saw all of them in their various oxidized states. When lumps of
each are only exposed to the air for a few hours, usually a deep blue oxide layer forms on La, Ce, and to a lesser extent, Pr and Nd, Pr and Nd are
still rather shiny after such time. After a few days on small pieces of the metal, they are simply a white powder. Never expierenced this with the
ingots,these were too precious to leave out.
I kept the filings from such work, and in a sealed vial they were storing nicely, untill the humidity in my basement changed and I was left with vials
of oxide   . The showed the same colour changes from shiny to blue to black to white, as
the degree of oxidation increased. . The showed the same colour changes from shiny to blue to black to white, as
the degree of oxidation increased.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Just out of curiosity, Might it be worthwile to store the Na metal under mineral oil with some kind of oxygen scavenger? Something (better than sodium
as far as scavenging oxygen is concerned) might be applied with an adhesive to the glass under Ar atmosphere. This would protect the metal from being
oxidized by left over oxygen after sealing.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Cerium.
Dark piece cut this last summer, grenish piece(brownish in the pic) old stock from the 80s, it was stored under some funky liquid with a strong smell.
I removed it and put it with this piece in mineral oil.
EDIT: I know I have said it before but rare earth ingots + bandsaw...YEEHAW
[Edited on 14-1-2007 by The_Davster]

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Blueish lanthanum, it is very air reactive compared to the other lanthanides, even under mineral oil(actually I think its ~50/50 mineral oil/hexane)
some whitish oxide can be seen falling off.

|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
My lanthanum has a beautiful dark blue oxide coating to it, and it's stored in a vial filled to the brim with boiled mineral oil and tightly sealed
with teflon. The cerium and praseodymium have developed a dark, hunter-green type coating to them while my neodymium has become nearly black. The
really weird thing is, the small areas on my neodymium which have oxidized into a powder turned into a light pink powder. It's really neat seeing the
contrast between the dark black surface and the occasional pink powder spot.
Now when I first saw the photo of the sodium with the blue coating, I immediately was reminded of the time that I saw sodium metal dissolved in
anhydrous ammonia. I now wonder if perhaps the outer electrons in your sealed sodium have become solvated in the ionic lattice of the incredibly thin
oxide layer, the glass, and the sodium metal? Could the blue colore be due to free electrons? Hmmmmmmm........
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
jamit
Hazard to Others
  
Posts: 375
Registered: 18-6-2010
Location: Midwest USA
Member Is Offline
Mood: No Mood
|
|
Most have suggested that sodium can be cleaned with xylene or toluene... But can it also be stored in these solvents.... Is it not stored under
toluene because of the risk of fire? I stored a small amount of sodium under toluene for about two weeks and the metal is really shiny... Beautiful
to see!
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
I have a small cylinder of welders argon which I use to flush containers with mineral oil in for storage of potassium and sodium. Before use I boiled
and cooled the mineral oil and left some small pieces of sodium in it to dry it completely. The freshly cut or melted metal is put in and the
container flushed with argon. I have potassium that looks pretty clean after months of storage. The lump of lanthanum is about 1cm by 2cm and has been
stored under argon. It looks bluer than in the picture.
  
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by nezza  | I have a small cylinder of welders argon which I use to flush containers with mineral oil in for storage of potassium and sodium.
|
For those of us who want to do a little occasional flushing, and don't want to invest in a 15 lb cylinder, the Bloxygen Finish Preserver looks good
($12.50 shipped):
http://www.hartvilletool.com/product/2323/miscellaneous-fini...
It appears to be pure argon:
http://complyplus.grainger.com/grainger/msds.asp?sheetid=376...
Each can contains 12 g of argon - which is 6.7 liters. It advertises "75 squirts". A "squirt" (90 mL) may be a reasonable amount for nearly full
container flush.
You can also fill a bottle of your choosing for your element collection.
[Edited on 31-8-2014 by careysub]
|
|
|
| Pages:
1
2
3 |