| Pages:
1
2 |
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I think the spiral is not actually attached to the column, thus just a simple column with a spiral thing stuffed inside, thus cheap to make. Might
work fine, might be so fragile that it breaks in first use, hard to know. But it is cheap, so not a big risk. Any type of plus that keeps the
packing in and does not clog the column is fine, but without insulation, the column will be hard to use well.
A really good jacketed packed or vigreaux column will cost more, but work better. It is a simple trade-off of quality verse price, which is often
not linear, so often spending a little more will benefit a lot, and then after that the cost of better goes up fast.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
A straight condenser with loosely packed fiberglass at the base and filled with rings does a very good job. I have also used broken glass tubing, you
don't want it too fine but too coarse doesn't work well either. Copper or stainless steel at the base will work for some substances, ie. not too
acidic.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
I have a couple of nice 55cm columns packed with stainless steel scourers which work pretty well.
The columns were supplied by SGL, product code CA12/33: http://scientificglass.co.uk/contents/en-uk/d61_Condensers_Air.html. Surprisingly cheap at £10.63 excl. VAT, and excellent quality.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Yes thats cheapish for a uk supplier but what was the P&P?
I recently purchased two Chines ones 300mm £10.38 each including P&P. Unfortunately the price of the longer ones jumps to £50+.
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
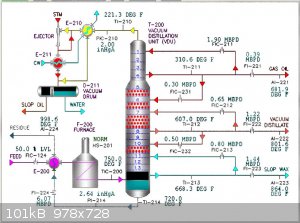
Does anybody know which type of column is this one?
It is speciffic,unusual,but not so uncommon,it has larger diameter in lower partm and it is narrow at the top.

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This may be a vacuum distillation column, or just an atmospheric distillation column. Here's a picture of the former, but one can find pictures of
atmospheric columns like it. Column design can be very specific to the feed and the intended products.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Anything that holds the packing in the column is fine. You want material with much surface area and little porosity, so it does not absorb the
liquid. broken thin glass, fine metal turnings, porcelin rings, etc are all fine.
If you don't have a vacuum jacketed column, you will want to insulate it on the outside, as otherwise it will cool too fast and not be very efficient.
The longer and wider the column, the better, but the larger the holdup. There is a real limit as to how pure you can get ethanol with one simple
column, it may take a few runs to get really good enrichment. There are companies that sell metal, insulated columns for distillaries/biofuel work
that work very well, but most are 4 feet tall and cost hundreds, but will handle a liter an hour or more. They take an enormous amount of heat
input, so a cheap hotplate won't work well. But for a smaller rig, like one liter, a good hotplate or mantle might work OK. For home brew, you are
only starting at about 10%, so you won't get more than 100 ml per L of input.
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
@Magpie
Maybe,maybe not
This is better picture,it was old linear alkyl benzene plant,no mention of vacuum column there,but who knows...

|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
I think this is exactly what @Magpie said.
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
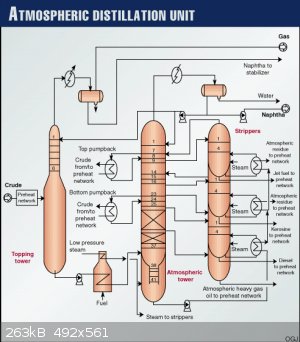
I am not sure,that's why i ask,here is atmospheric distillation...
So other columns are not at atmospheric pressure?Why that construction?
P.S. exactly this tower is shown as "topping tower" at this picture,what's that exactly?
I know this is small aparatus forum but maybe some process engineer answers 
[Edited on 1-12-2018 by sulfuric acid is the king]
[Edited on 1-12-2018 by sulfuric acid is the king]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, I too would like to hear from process engineers with experience with distillation columns.
I am a retired process engineer (ChE) but never had the opportunity to work with distillation columns. So my comments are based only on what I
learned 54 years ago in a class called Unit Operations which covered distillation.
I would say that the "topping tower" is being fed heated crude oil. Off the top is gas (methane, ethane, propane, etc) and some naphtha. The bottoms
off this tower are again heated then go to the bottom of the atmospheric distillation column shown in the middle of the diagram. Most of the
fractionation takes place there.
A little searching revealed what I suspected: the wide cross-sections of columns are found where the gas rates are high.
[Edited on 2-12-2018 by Magpie]
[Edited on 2-12-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I guess that for a given throughput at lower pressures
there will be very high gas/vapour velocities due to the high molar volumes, P.V = R.T etc.
unless much wider towers are used.
..........
How could you see the difference between a high throughput high pressure tower compared to a low throughput low pressure tower ?
[Edited on 2-12-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
| Pages:
1
2 |