| Pages:
1
2 |
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
fdsailor, I have no idea where you've found the stupid guides with bubbling HCl through water, but the right way is to always have
the inlet tube above surface of receiving solution.
|
|
|
Deathunter88
National Hazard
   
Posts: 519
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  | | fdsailor, I have no idea where you've found the stupid guides with bubbling HCl through water, but the right way is to always have
the inlet tube above surface of receiving solution. |
Um, IDK about you but all the sources I have read suggest bubbling the HCl into the water as it makes the process more efficient...Just be sure to
plan for suck back by having a second flask.
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
Use an inverted funnel, placed just under the surface of the water, to avoid suck-back. This is an old trick and it works very well, also with
ammonia.
Design it so that as the water starts to rise in the funnel, it lowers the level of the water outside the funnel until it breaks loose and lets air
in. In my experience you get very little of the soluble gas (HCl or NH3) coming out into the room, even when the solution is near saturation.
Any other SF Bay chemists?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Salt, vinegar and bleach does that, as everyone knows.
https://www.sciencemadness.org/whisper/viewthread.php?tid=63...
Aqua regia also works.
Chlorine too.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Just use a funnel-and-beaker trap and forget about suckback.
Smells like ammonia....
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Suck-back is always something to consider in this type of generator/gas/liquid arrangement.
The inverted funnel is an excellent idea. and works very very well.
|
|
|
crackedbits
Harmless

Posts: 3
Registered: 1-3-2018
Member Is Offline
Mood: No Mood
|
|
I found this forum and more specifically this thread, because I'm wanting to concentrate the store bought Muriatic Acid which is in the 30% to 34% by
volume range, to the reagent level of 36% to 38% concentration by volume. I'm an amateur chemist and have a small lab setup, but I'm not sure of what
other equipment I may need to make this endeavor more efficient and repeatable. I'm not looking to make gallons of the stuff, but a process that
yields any where from 500ml to 1L would be nice. I've seen the method by NurdRage on Youtube and a few other examples, nothing of which gives me a
comprehensive layout of what equipment and expected yield. Any help would be appreciated, thanks.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
In my experience, hydrochloric acid is easier synthesized from scratch than bought and purified.
To make azeotropic HCl, I use table salt and battery acid (36% sulfuric acid). I mix table salt and battery acid in a flask and start distilling the
mixture. Azeotropic HCl comes over, along with a small amount of HCl gas. Since both reagents are very cheap, this method can be used to distill large
amounts of 20% HCl.
To make concentrated HCl, I use boiled-down battery acid. I don't "boil the bat" all the way down to 95%, 70% is more than enough for making HCl gas.
Boil the battery acid until it starts fuming, then cool it. Put table salt in a flask, pour sulfuric acid in, stopper the flask quickly and start
heating it, trapping the resulting HCl gas in azeotropic HCl you made previously using a funnel and beaker trap. This way you can make concentrated
HCl all the way to 36%.
Smells like ammonia....
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
@crackedbits
It seems to me that you want to bump up the concentration a little bit. I am not sure why. Fuming of HCl increases greatly as you raise the
concentration into the thirties. I am not sure what the solubility limit is but it can't be too far from teh azeotrope.
My approach would be to chill the muriatic acid as low as it will go. Then produce HCl gas via NaCl and concentrated sulfuric acid. Bubble this
through your chilled muriatic acid. Keep a thermometer in there so that hopefully you notice when no more gas is dissolving. Pass the excess gas
through a suckback trap followed by iced water to avoid rusting everything in your fumehood.
|
|
|
crackedbits
Harmless

Posts: 3
Registered: 1-3-2018
Member Is Offline
Mood: No Mood
|
|
HCL Concentration Setup
I was thinking of something along these lines, based on what I've seen and some suggestions.
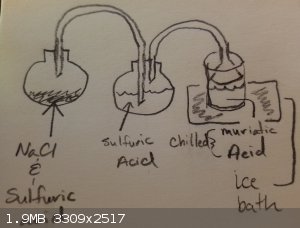
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
What is the second flask for? Drying HCl gas? Why do you want to dry it, if you dissolve it in water anyway?
Smells like ammonia....
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
When I want to avoid suckback in a distillation, I attach a vacuum adapter to the receiving flask and put on a piece of rubber tubing which I kink
using my hand. As the solution rises up the tube, the kink is relaxed and atmospheric pressure is restored. Probably not as suitable for large
distillations, but it’s always worked perfectly well for <200mL quantities. The only times suckback has been too rapid is when the solution is
allowed to climb to the top of the tube and reaches the horizontal (I use a long ~80 degree distillate receiver to bubble gases), but before this
point it maxes out at about 1cm per second and that’s near the end of the reaction when heat is taken off, so plenty of warning to take action.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
My HCl is 36% because that's what is commonly available here,
I can't think of aan occasion that I needed 36% concentration, even for aqua-regia.
I've even considered diluting my 36% to Azeotropic to reduce the rate at which my tools rust.
So my question has to be - why do you want highly concentrated hydrochloric acid ?
or, is there any good reason to keep my HCl at 36% instead of diluting to 20% ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
crackedbits
Harmless

Posts: 3
Registered: 1-3-2018
Member Is Offline
Mood: No Mood
|
|
@ave369; Yes, I'm not wanting to add anymore water to my end product so I dry it through the second flask with sulfuric.
@LearnedAmateur; thanks for the tip.
@Sulaiman; I can actually buy ACS grade HCL, but it's six times the price of Muriatic, I have equipment, so I figured, I'd save myself some money. I'm
also wanting to recycle the HCL I use in other processes. Bottom line, I want to save money, using less chemicals and recycling.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
An easy purification of muriatic acid is distillation,
if the starting concentration is greater than azeotropic
then the first hydrochloric acid to distill over will be more concentrated than the starting muriatic acid,
until azeotropic hydrochloric acid distills over.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here an extract from some related comments on increasing the strength of HCl I once did:
Quote: Originally posted by AJKOER  | .......
........
In the case of dilute HCl where the addition of a salt is not problematic for a particular application, one may consider the addition of anhydrous
calcium chloride (or, a concentrated solution thereof) as a possibility. The reason relates to the apparent significant increase in the so called
'activity level' upon adding MgCl2 or CaCl2 or NaCl (in declining order of preference). Here is a real world reference relating to practical
significance in the field of Hydrometallury where leaching out minerals from ores efficiently and cheaply is a major concern. Source: See
"Hydrometallurgy in Extraction Processes", Vol I, by C. K. Gupta and T. K. Mukherjee, page 15 at http://books.google.com/books?id=F7p7W1rykpwC&pg=PA15 .
Note, the author claims there is data confirming that a 2M HCl in 3M MgCl2 or CaCl2 (and also FeCl3) behaves like 7M HCl!
Here is a quote on the matter of discussion in one of the reference sources previously provided by Bfesser on Thermodynamic Activity (see http://en.wikipedia.org/wiki/Thermodynamic_activity ):
"When a 0.1 M hydrochloric acid solution containing methyl green indicator is added to a 5 M solution of magnesium chloride, the color of the
indicator changes from green to yellow—indicating increasing acidity—when in fact the acid has been diluted. Although at low ionic strength
(<0.1 M) the activity coefficient approaches unity, this coefficient can actually increase with ionic strength in a high ionic strength regime. For
hydrochloric acid solutions, the minimum is around 0.4 M.[1]"
[Edited on 22-10-2014 by AJKOER] |
Link: http://www.sciencemadness.org/talk/viewthread.
---------------------------------------------------------------
Interestingly, depending on the intended use of the HCl, say in organic formations of chlorides, an interesting idea is perhaps not aiming so much at
concentrating the HCl itself, but instead focus on the formation of the monoatomic chlorine radical to effect reactions. This could proceed by adding
some Cl2 to the HCl along with UV light and warming. Some chemistry on the proposed HCl/H2O/Cl2/UV mix::
Cl2 + H2O = HCl + HOCl
HOCl + heat → HCl + 1/2 O2
Cl2 + Cl-(aq) = Cl3-(aq) (or perhaps, Cl2 + HCl(aq) = HCl3(aq) )
HOCl + hv = OH• + Cl•
OH• + Cl- = OHCl•-
OHCl•- + H+ → H2O + Cl• (see http://pubs.acs.org/doi/abs/10.1021/j100497a003 )
Cl2 + hv = Cl• + Cl• (gas phase reaction is more efficient at higher pressure)
Cl3-(aq) + hv =?= 2 Cl• + Cl- (as the existence of Cl3- is itself speculative, likely unstable, and may be more effective than the prior gas phase
reaction of gaseous Cl2)
Or: HCl3 (aq) + hv =?= 2 Cl• + HCl (speculation, but as the very existence of HCl3 is questioned, it lack of stability on irradiation seems
plausible)
Cl• + Cl- = Cl2•- (this radical has increased longevity over Cl• but lower reactivity)
Additionally, one can increase the hydroxyl radical concentration (leading to Cl•) via the use of N2O and UV light:
N2O + H2O + UV --> N2 (g) + •OH + OH-
The hydroxyl radical can also be sourced from electrochemical redox or fenton/fenton-type reactions. See comments in hydrometallurgy reference above.
Example of applications:
CH3OH + hv --> •CH3 + •OH
•CH3 + Cl• --> CH3Cl
CH4 + Cl• --> •CH3Cl + HCl
However, some reactions which may not be desirable depending on the intended use of the HCl/H2O/Cl2/UV mix, introducing a range of products:
OH• + ROH --> H2O + •RO
......
Also from the reaction:
Cl• + HOCl = HCl + ClO• (see, for example, http://pubs.acs.org/doi/abs/10.1021/acs.jpca.5b01273 )
........
where the ClO• may further introduce chloride product impurities.
If your dilute HCl is also impure, a much better idea may be to convert it into chlorine and proceed similarly per above with added UV.
[Edited on 3-3-2018 by AJKOER]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Found an example of the application of chlorine radical formation in chlorine water with added NaCl and Fe(lll)/Fulvic acid as a photo-fenton source
of hydroxyl radicals, OH•, and resulting Cl• and with Cl-, Cl2•− radicals. To quote: "Formation of Chlorinated Intermediate from Bisphenol A
in Surface Saline Water under Simulated Solar Light Irradiation", by Hui Liu, Huimin Zhao, Xie Quan, Yaobin Zhang and Shuo Chen, Environ. Sci.
Technol., 2009, 43 (20), pp 7712–7717, DOI: 10.1021/es900811c .
"Synopsis
Photoformation of organochlorine compound in surface saline water is demonstrated as one of its natural sources, and its formation mechanism is
explained.
Abstract
Chlorinated organic compounds are generally of great concern, but many uncertainties exist regarding how they are generated. To illustrate the
possibility of photochemical formation of organochlorine compounds in natural water, the phototransformation of bisphenol A (BPA) in aqueous saline
solution containing Fe(III) and fulvic acid (FA), and in coastal seawater under simulated solar light irradiation was investigated.
2-(3-Chloro-4-hydroxyphenyl)-2-(4-hydroxyphenyl) propane (3-ClBPA) and 2,2-bis(3-chloro-4-hydroxyphenyl) propane (3,3-diClBPA) were the main
chlorinated derivatives during the processes. Laser flash photolysis (LFP) and electron spin resonance (ESR) results indicated that the chlorination
of BPA was most likely due to the formation of Cl2•− radical as a consequence of Fe(III) irradiation, yielding Cl• and OH• radical species and
finally forming Cl2•− radical upon further reaction with chloride. The formation of Fe(III)−FA complex, which is a normal coexistence
configuration of Fe(III) and FA in natural water, promoted the BPA chlorination through producing more Cl2•− radical. Moreover, FA had two
opposite effects: forming Fe(III)−FA complex to enhance Cl2•− formation and competing radicals with BPA, which resulted in different overall
effects at different concentrations: BPA chlorination was enhanced with the increasing of FA concentration ([FA]) when [FA] < 3.2 mg L−1; when
the concentration of FA was as high as 10 mg L−1, it slowed down obviously. The described BPA photochlorination process took place from pH 6.3 to
8.5 and increased with the increasing of chloride concentration, indicating it could occur universally in natural saline surface water. These results
propose a natural photochemical source for organochlorine compounds."
Link: https://pubs.acs.org/doi/abs/10.1021/es900811c
----------------------------------------------------------------
Here is an old work citing the historic known action of chlorine on benzene in the UV light available at: https://smartech.gatech.edu/bitstream/handle/1853/27667/fowl... .
[Edited on 8-4-2018 by AJKOER]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
HCl gas doesn't seem to suckback nearly as strongly against an azeotropic solution as against pure water. The suckback vs. water is pretty ferocious.
|
|
|
| Pages:
1
2 |