| Pages:
1
2 |
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Have you thought about Biuret perchlorate? Biuret can be easily made by heating urea, so why not to try it?
Rest In Pieces!
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Here is a paper describing biuret perchlorate detection.
Biuret Perchlorate
It is pretty strange that oxygen is protonated instead of NH2 group.
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The oxygen would be protonated rather than an amine group because the amine group is electron donating to the oxygen.
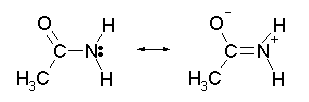
This is the reason why amides groups are so much less reactive than ketones, aldehydes, or amines.
[Edited on 27-1-2012 by AndersHoveland]
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Urea Perchlorate seems interesting material. it has much Nitrogen. I made a search for it but couldnt find much info rather than :

any more info about its properties ?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Urea perchlorate (and also urea nitrate) are very impractical energetic materials because of their high acidity and simultaneous hygroscopicity... a
really bad combo. Urea is a very weak base with a pKb(BH+)=0.18 (wikipedia), so it only forms salts with strong acids and those salts are themselves
very acidic indeed.
In some respect, urea perchlorate can be viewed as a source of anhydrous perchloric acid, i.e. because of the ease with which it can be deprotonated.
Since perchloric acid forms dangerous and sensitive explosives with a wide range of materials, this is a further reason that this perchlorate hasn't
made it into mainstream use.
Indeed as has been stated earlier on in this thread, the related guanidinium perchlorate, being the salt of a strong base and acid, is a MUCH better
way to go.
|
|
|
specialactivitieSK
Hazard to Self
 
Posts: 94
Registered: 21-10-2014
Member Is Offline
Mood: No Mood
|
|
Better is Guanidine Perchlorate formed by reacting Sodium Perchlorate with Guanidine Hydrochloride.
Non-hygroscopic, moderately sensitive to impact and has considerable power.
1,15 g/ccm = 6000 m/s
400 cm3
It's Explosive appropriate force tetryl.
[Edited on 1-10-2015 by specialactivitieSK]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Even better would be:
-Biguanidine diperchlorate
H2N-C(=NH)-NH-C(=NH)-NH2 . 2 HClO4
-Pentaerythritamine tetrapechlorate
C(-CH2-NH2)4. 4 HClO4
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by PHILOU Zrealone  | Even better would be:
-Biguanidine diperchlorate
H2N-C(=NH)-NH-C(=NH)-NH2 . 2 HClO4
-Pentaerythritamine tetrapechlorate
C(-CH2-NH2)4. 4 HClO4 |
unfortunately, I didnt find any info about both materials on google.
|
|
|
specialactivitieSK
Hazard to Self
 
Posts: 94
Registered: 21-10-2014
Member Is Offline
Mood: No Mood
|
|
Biguanide diperchlorate and process for preparation thereof
US 4340755 A :
http://www.google.com/patents/US4340755
Would it be possible, by reaction Biguanide hydrochloride with sodium
perchlorate ?
[Edited on 16-10-2015 by specialactivitieSK]
[Edited on 16-10-2015 by specialactivitieSK]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes possible.
Also another interesting possibility:
H2N-C(=NH)-C(=NH)-NH2 . 2 HClO4
the diperchlorate of the diimino derivative of oxalamide
[Edited on 16-10-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I could synthesis now perchlorate and I would like to go further and make Biguanide diperchlorate !
sorry if my question looks very basic but i found very hard ways to synthesis Biguanide
isn't there some practical steps to be synthesis Biguanide at home in my small lab?
|
|
|
| Pages:
1
2 |