| Pages:
1
2 |
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
The procedure for synthesizing sodium hydride from sodium metal can be found in Inorganic Syntheses Collective Volume V on pages 6-13. The procedure
details the production of an extremely fine dispersion of sodium metal and the subsequent reaction with hydrogen between 220 C -280 C at atmospheric
pressure in standard laboratory glassware. This could be of use if sodium metal is available since sodium hydride can be reacted with methyl borate to
form sodium borohydride.
Amateur NMR spectroscopist
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Actually at STP (or anywhere near it) the only product found by Schlesinger/Brown was Sodium Trimethoxyborohydride, the reaction of that in THF with
Diborane is pure sodium borohydride though. At high temperature and pressure, NaH does react to form "some" borohydride. I'm waiting on the reference,
but that is what the first page says.
The file attached "isn't" the one I was thinking of, but it does show the use of solvent for the preparation of Lithium Borohydride, Sodium
Trimethoxyborohydride and from THAT in solvent with diborane, one can achieve Sodium Borohydride.
[Edited on 2-8-2010 by un0me2]
Attachment: Brown.Tierney.The.Reaction.of.Lewis.Acids.of.Boron.with.Sodium.Hydride.and.Borohydride.pdf (947kB)
This file has been downloaded 1662 times
quam temere in nosmet legem sancimus iniquam
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Here is me looking at triethylborane and looking to see what is the minimum level of oxygen with which it reacts, only to find these articles (here, here & here) in which they researched the radical reactions of triethylborane-air @ 20C. Fair enough, they did only open the things up after the reaction
had started (and everything was in solution), but that would have took balls, BIG BALLS - they also cite this paper where 600mL was good for 16 restarts/afterburner lighting on the SR-71 Blackbirds Combined Operation Turbojet/Ramjet-type engines. - they also cite this paper where 600mL was good for 16 restarts/afterburner lighting on the SR-71 Blackbirds Combined Operation Turbojet/Ramjet-type engines.
Radical reactions? What like people running like fuck when they realise what the clown in the next booth is going to do?
[Edited on 4-8-2010 by un0me2]
quam temere in nosmet legem sancimus iniquam
|
|
|
Chainhit222
Hazard to Others
  
Posts: 138
Registered: 22-8-2009
Location: peach's mailbox
Member Is Offline
Mood: grignard failing to start
|
|
Quote: Originally posted by benzylchloride1  | | The procedure for synthesizing sodium hydride from sodium metal can be found in Inorganic Syntheses Collective Volume V on pages 6-13. The procedure
details the production of an extremely fine dispersion of sodium metal and the subsequent reaction with hydrogen between 220 C -280 C at atmospheric
pressure in standard laboratory glassware. This could be of use if sodium metal is available since sodium hydride can be reacted with methyl borate to
form sodium borohydride. |
here you go:
http://img231.imageshack.us/img231/6498/sodium1.jpg
http://img571.imageshack.us/img571/8318/sodium2.png
http://img155.imageshack.us/img155/3707/sodium3.jpg
http://img641.imageshack.us/img641/7985/nah1.jpg
http://img375.imageshack.us/img375/3885/nah2.jpg
http://img641.imageshack.us/img641/5019/nah3t.jpg
http://img52.imageshack.us/img52/2431/nah4.png
http://img8.imageshack.us/img8/30/nah5.jpg
[Edited on 4-8-2010 by Chainhit222]
The practice of storing bottles of milk or beer in laboratory refrigerators is to be strongly condemned encouraged
-Vogels Textbook of Practical Organic Chemistry
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
It is probably easier for all if you just print it - use PrimoPDF as a printer, then just print the pages - to get the effect you see in the attached
PDF's, one is the same preparation of Sodium Dispersions and the other the preparation of Lithium Hydride (in a glass tube with a Mecker burner
@500C).
Now I'm wondering, has anyone seen the preparation of LiH in dispersion? I know I've seen papers on making Lithium Sand in order to make Alkyllithium
reagents, much the same as the sodium dispersion is made. So it strikes me that it should be possible to prepare LiH in much the same way as is done
with the sodium dispersion... Albeit at a higher temperature maybe, but surely it would react?
Attachment: Inorganic.Syntheses.Vol.5.pp.6.10.Sodium.Dispersions.and.Sodium.Hydride.pdf (274kB)
This file has been downloaded 1039 times
Attachment: Vorobyova.Practical.Inorganic.Chemistry.1987.pp.184.5.Preparation.of.Lithium.Hydride.pdf (93kB)
This file has been downloaded 802 times
quam temere in nosmet legem sancimus iniquam
|
|
|
Chainhit222
Hazard to Others
  
Posts: 138
Registered: 22-8-2009
Location: peach's mailbox
Member Is Offline
Mood: grignard failing to start
|
|
Quote: Originally posted by un0me2  | It is probably easier for all if you just print it - use PrimoPDF as a printer, then just print the pages - to get the effect you see in the attached
PDF's, one is the same preparation of Sodium Dispersions and the other the preparation of Lithium Hydride (in a glass tube with a Mecker burner
@500C).
Now I'm wondering, has anyone seen the preparation of LiH in dispersion? I know I've seen papers on making Lithium Sand in order to make Alkyllithium
reagents, much the same as the sodium dispersion is made. So it strikes me that it should be possible to prepare LiH in much the same way as is done
with the sodium dispersion... Albeit at a higher temperature maybe, but surely it would react? |
I bet you that it will work just for for LiH. You can use the hydrogen test described in the NaH procedure to see if its working (compare two
bubblers, if output is bubbling slower then input then it will be turning into LiH)
[Edited on 5-8-2010 by Chainhit222]
The practice of storing bottles of milk or beer in laboratory refrigerators is to be strongly condemned encouraged
-Vogels Textbook of Practical Organic Chemistry
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Well the alternative is always the preparation then the thermal degradation of alkyl (ethyl) lithium to ethylene and LiH, which is about as short a
route (wasteful though - 50% loss off the bat) as probably exists. Although doing so directly from ethyllithium, as in the attached paper (cited many,
many places (such as here & having been reported by Schlesinger & Brown, I'll take them at their word - it ain't some dodgy patent).
The second paper (attached) catalytic degradation of Ethyllithium into LiH + ethylene (good only if you are mad enough to still want triethylborane
and/or super hydride after reading the other paper).
[Edited on 5-8-2010 by un0me2]
Attachment: Schlesinger.Brown.Metallo.Borohydrides.III.Lithium.Borohydride.pdf (181kB)
This file has been downloaded 1601 times
Attachment: Zgonnik.etal.Reactions.of.Organometallic.Compounds.with.HeavyMetal.Salts.4.Reaction.of.Ethyllithium.with.Titanium.Trichl (108kB)
This file has been downloaded 918 times
quam temere in nosmet legem sancimus iniquam
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
sodium borohydride from common reagents
CH4 + NaBO2 Heat -> NaBH4 + CO2
Sodium metaborate (borax can be used) is strongly heated in an atmosphere of methane (in home natural gas is 97% methane) which produces sodium
borohydride and carbon dioxide. The suggested reaction temperature is 900C. I'm thinking I'll use a stainless steel vessel with an inlet tube
extending to just above the borax melt. As the sodium borohydride is formed it will boil and I should be able to condense it with a coldfinger. The
vessel will have a an exit port at the top for purging unreacted natural gas which will sweep away the CO2. The reaction vessel will look something
like this:
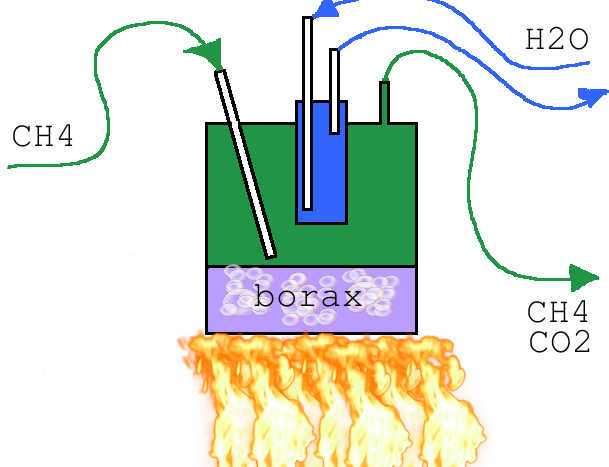
I have searched the forum and do not see any other chatter about the aforementioned reaction. Has anyone here attempted this synthesis? If anyone has
any details, references, or comments about this reaction I'd love to read them. Here's what I could dig up with patent searches and google.
Attachment: methane.borax.to.sodium.borohydride.us7019105.pdf (184kB)
This file has been downloaded 973 times
Attachment: methane.borax.to.sodium.borohydride.us7294323.pdf (72kB)
This file has been downloaded 894 times
Attachment: millennium.cell.inc.review.chemical.processes.pdf (404kB)
This file has been downloaded 1942 times
Attachment: us.dept.of.energy.fuels.of.the.future.for.cars.and.trucks.pdf (502kB)
This file has been downloaded 851 times
|
|
|
wireshark
Harmless

Posts: 28
Registered: 21-1-2013
Member Is Offline
Mood: No Mood
|
|
I've skimmed the third document before. Look at it again. The ΔG using methane is too positive for this reaction to proceed "at any reasonable
temperature."
If you can't get borohydride, use reductive hydrogenation. Often the point of using metal hydrides is convenience, so it's pretty ridiculous to try to
make them. Anyway, metal hydrides are impractical for industry and the chemical-lacking chemist alike.
[Edited on 3-2-2013 by wireshark]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Not all industry. The pulp bleaching industry uses NaBH4, probably by the ton. See Wiki. Also see the very informative publication by Rohm &
Haas linked in the Wiki article references.
Forum member ordenblitz thought he had stumbled on a facile method for the home chemist but he dropped his research. I tried to pick up where he left
off but also had no success.
[Edited on 3-2-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
And I did it just now, as it's one of few non-patent google hits mentioning the MgB2 -> KBH4 process of JACS 78, 4176 (1956) and any patents they
wrote. People here are clearly on to this but I couldn't find any actual mention of the little
article...http://pubs.acs.org/doi/abs/10.1021/ja01597a093
"A 13% conversion of boron to borohydride was obtained, as determined by the amount of hydrogen evolved upon acidification of the solution."
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by S.C. Wack  |
And I did it just now, as it's one of few non-patent google hits mentioning the MgB2 -> KBH4 process of JACS 78, 4176 (1956) and any patents they
wrote. People here are clearly on to this but I couldn't find any actual mention of the little
article...http://pubs.acs.org/doi/abs/10.1021/ja01597a093
"A 13% conversion of boron to borohydride was obtained, as determined by the amount of hydrogen evolved upon acidification of the solution."
|
13% sounds very good if this method really works, looks pretty easy, almost too easy, in fact.
|
|
|
Orenousername
Hazard to Self
 
Posts: 79
Registered: 16-4-2016
Location: USA
Member Is Offline
Mood: Regulated
|
|
Has anyone tried MgH2 + NaBO2 yet? and do the reagents need to be anhydrous?
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Sure, this has been done, but probably not by anyone here. And, yes....the reagents would have to be completely anhydrous.
Also do-able with CaH2, which was formerly an inexpensive and easy get. MgH2 works better though,
Check via the search engine. There is a lot of information on this topic.
[Edited on 24-4-2016 by zed]
|
|
|
CRUSTY
Hazard to Others
  
Posts: 139
Registered: 5-6-2016
Location: Nearby
Member Is Offline
Mood: High-Order
|
|
I would assume they need to be anhydrous, since NaBH4 reacts with water. I would try this, but MgH2 seems like a total pain in
the ass to work with, as you'd need to run the hydrogenation (as well as the MgH2 synthesis) under an inert, anhydrous atmosphere, and you
have to some sort of milling setup and a very fine magnesium powder in order to get a reasonable yield, since H2 gas can barely diffuse
through MgH2 IIRC.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I don't think anyone has successfully produced the hydrides. Everyone focuses on what you do after you get the hydride because nobody can figure out
how to make one lol.
It's known that magnesium in napthalene containing catalytic -- [TiCl4 or TiBr4] EDIT: CrCl3 works which is WAY better -- can be hydrogenated at 20 C
at normal pressure.
http://onlinelibrary.wiley.com/doi/10.1002/anie.198008181/fu...
I think this is probably going to be the type of procedure that eventually succeeds. The ball milling procedure mentioned on page 1 is touchy and
requires activated magnesium in a perfectly dry atmosphere. TiCl4/naphthalene acts as a powerful in situ dehydrating system to get the reaction going
that's just more practical... it's possible that the requisite catalyst loading is small.
Bromine is dangerous, but the reaction of a tiny amount of bromine with titanium can't be too bad, right? :p
The trimethoxyaluminum hydride ion can then be made (I think) by reaction of a metal hydride with Al(OMe)3. The latter is made by simply
reacting activated aluminum with methanol, i.e. Al + 3 MeOH + cat HgCl2 >> Al(OMe)3 + 3/2 H2. Magnesium trimethoxyaluminum hydride is the
presumptive reaction product of MgH2 + Al(OMe)3.
https://en.wikipedia.org/wiki/Reductions_with_metal_alkoxyal...
EDIT: Actual paper attached. CrCl3 instead of TiCl4 is a *big* improvement. EDIT2: FeCl2 is even better.
Attachment: 10.1002@anie.198008181.pdf (246kB)
This file has been downloaded 638 times
[Edited on 13-6-2016 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
"Bromine is dangerous, but the reaction of a tiny amount of bromine with titanium can't be too bad, right? :p"
Bromine is dangerous, but we used to routinely use it in lower level chemistry courses. I can remember a classmate getting bromine all over his
fingers. He got it off right away, and as I recall, there were no serious consequences.
I might be concerned about Bromine reacting with Titanium. Titanium is pretty non-reactive, but once it decides to react, it might not be easy to put
the brakes on.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The article also mentions that FeCl2 can be used as a catalyst. Assuming this is correct (and doesn't actually refer to FeCl3), anhydrous FeCl2 can be
produced using methanolic HCl with vacuum drying:
https://en.wikipedia.org/wiki/Iron(II)_chloride#Laboratory_preparation
This is probably the easiest way to make one of the transition-metal catalysts. I don't think it was a typo, either -- FeCl3 seems too oxidizing to be
used in the preparation of a hydride.
NB: the produced MgH2 is pyrophoric!
[Edited on 13-6-2016 by clearly_not_atara]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
For anyone interested, here is the article referenced by the wiki page :
Attachment: FerrousHalides.pdf (37kB)
This file has been downloaded 565 times
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Found this and thought I'd drop it here............
"Nanocrystalline magnesium for hydrogen storage"
https://doi.org/10.1016/S0925-8388(99)00073-0
Abstract
The hydrogen storage properties of MgH2 are significantly enhanced by a proper engineering of the microstructure and surface. Magnesium powders are
produced in a nanocrystalline form, which gives remarkable improvement of absorption/desorption kinetics. Ball milling, which is used for fabrication
of nanocrystalline magnesium, improves both the morphology of the powders and the surface activity for hydrogenation. The hydriding properties are
further enhanced by catalysis through nano-particles of Pd located on magnesium surface. Nanocrystalline magnesium with such a catalyst exhibits an
outstanding hydrogenation performance: very fast kinetics, operation at lower temperatures than conventional magnesium and no need for activation.
And still using the ball mill............
"Preparation of sodium borohydride by the reaction of MgH2 with dehydrated borax through ball milling at room temperature"
https://doi.org/10.1016/S0925-8388(02)00872-1
Abstract
A convenient method was developed to synthesize NaBH4 by the reaction of MgH2 with Na2B4O7 through ball milling at room temperature. In order to
improve the sodium borohydride yield, Na compounds were added to compensate the Na insufficiency in reactants when MgH2 instead of NaH was used as the
reducing agent. It was found that Na2CO3 addition was better than NaOH or Na2O2 addition in increasing the borohydride yield.
/CJ
[EDIT] - Attachment
[EDIT2] - Attachment2
[Edited on 5-12-2017 by Corrosive Joeseph]
Attachment: li2003.pdf (551kB)
This file has been downloaded 506 times
[Edited on 5-12-2017 by Corrosive Joeseph]
Attachment: zaluska1999.pdf (630kB)
This file has been downloaded 537 times
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by S.C. Wack  |
And I did it just now, as it's one of few non-patent google hits mentioning the MgB2 -> KBH4 process of JACS 78, 4176 (1956) and any patents they
wrote. People here are clearly on to this but I couldn't find any actual mention of the little
article...http://pubs.acs.org/doi/abs/10.1021/ja01597a093
"A 13% conversion of boron to borohydride was obtained, as determined by the amount of hydrogen evolved upon acidification of the solution."
|
We should contextualize this. I believe the balanced reaction should be:
4 KOH + 2 MgB2 >> 2 MgO + KBO2 + KBH4 + 2 B
So the ideal yield is only 25% and 13% molar yield is 52% of theory.
The remaining boron can be recovered and reused quite feasibly if the solution is acidified since we should see precipitation of boric acid and of
course elemental B is not soluble.
The last question is how to optimize the reaction:
Mg + B + B2O3 >> MgO + MgB2
to obtain a reaction which is self-sustaining but not too exothermic and makes good use of the Mg. I suspect that the product mixture can be separated
by using cold aqueous acetic acid solution to dissolve MgO without much decomposition of MgB2.
[Edited on 9-11-2023 by clearly_not_atara]
|
|
|
| Pages:
1
2 |