| Pages:
1
2
3
4
5 |
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
I have thought about making catechol myself from methyl salicylate using the Bouveault Blanc reduction http://en.wikipedia.org/wiki/Bouveault-Blanc_reduction... I do not have any literature references for this, I have talked to a professor about it
though, they said that the Lithium or sodium in ethanol should not be strong enough to reduce the benzene ring.
any reason or something that I am ignorant to....as to why this reaction will not work or has not been suggested for making catechol?
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Here is the reference to a paper about the reaction.. it mentions that aromatics are not reduced and phenols and Carboxylic acids were tolerated.
http://pubs.acs.org/doi/pdf/10.1021/jo802778z
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Well for a start, bouvealt-blanc reduction will take the ester to the alcohol. All gravy, but then you've got a benzylic alcohol and not a phenol. I
suppose oxidation to the aldehyde and subsequent Dakin reaction would work but it sounds like alot of hassle to me.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
This entire thread depends on why you're doing it.
If it's for the fun of it and to get a pure reference, yeah, go heavy duty on the ground up method.
If it's for a bit of fun, use one of the naturally occurring close variants like guaiacol and demethylate it. Blasting it with a hard lewis acid will
be fairly simple compared to other demethylations.
If it's to get something working, just buy a bottle of the crap. The photography guys have some if you don't have a supplier account.
If it's a scheme to an unlimited supply of drugs.... it's probably not going to happen. Acid and cocaine can both be made from very basic beginnings.
The 12 step process and 1% yield, involving some seriously tricky chemistry along the way, means no one has bothered. Despite people wanting them
enough to kill each other.
[Edited on 13-10-2010 by peach]
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
"subsequent Dakin reaction" after oxidation to the aldehyde...
yes yes.. sorry
I agree
sorry idk how that even slipped my mind, and I was even TAing organic lab at the time I feel..blegh XD
[Edited on 14-10-2010 by ThatchemistKid]
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
well.. wait a moment that is counter productive.. can the bouvealt-blanc reduction not be adjusted to give the aldehyde?
It takes two equivalents of the alkali metal to reduce the acid all the way to the alcohol. an intermediate in the mechanism is the aldehyde, can it
not be stopped there if the conditions are controlled.. say a little excess Salicylic acid to only about 1 equivalent of alkali-metal? ... essentially
we would be going through Salicylaldehyde as mentioned in some of the other procedures here but we would be skipping the Reimer-Tiemann formylation (
if phenol or benzene was your starting material) and going straight to the dakin reaction.
It would also avoid the salicylamide formation and the hoffman degradation and subsequent diazotization.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nope - think electrophilicity of the species in question. Its the same reason LAH reduction of esters can't be stopped at the aldehyde.
[Edited on 14-10-2010 by DJF90]
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Well is there a milder reducing that will take the ester only to the aldehyde?
hopefully ones that are more common and accessible than diisobutylaluminum hydride? ( which is done at -70C), although if this last method is of
interest to anyone I have uploaded here a good paper that I found about the subject.
And I figured that the electrophilicity might be an issue as with LAH reductions but I have no experience as of yet with the reaction so I did not
actually know.
Attachment: Dibalh salts reduction of esters to aldehydes.pdf (354kB)
This file has been downloaded 1105 times
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
DIBAL-H and the salts that paper mentions (which work far better than DIBAL-H itself for the Ester=>Aldehyde transformation) are typically what are
used, and I'm not sure there is another *direct* alternative. LAH reduction of the corresponding acyl aziridines, acyl imidazoles, or weinreb amides
should also furnish the aldehyde. Take your pick, but its all alot of hassle for a commercially available material.
|
|
|
ThatchemistKid
Hazard to Others
  
Posts: 132
Registered: 2-6-2010
Member Is Offline
|
|
Note by common and accessible I meant to the home amature XD
although I do know that the synthesis of LAH and its derivatives is not to terribly difficult that someone with a little skill, and possibly a
glovebox, may achieve it at home...I have yet to do that...as I do not think I am that skilled yet.
[Edited on 15-10-2010 by ThatchemistKid]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Then the Bouvealt-Blanc is probably the best bet. Catechol should still be accessible to you though, especially if you can get sodium.
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Just wanted to pop in and comment on this necromantic thread.
Seems to me that to go from salicylic acid to catechol, the simplest route would be:
1. Mix with CaOH, heat and decarboxylate to phenol.
2. Treat phenol with Fenton's Reagent to generate a mixture of dihydroxybenzenes.
3. Separate via vacuum distillation.
Found a rather detailed site on Fenton's Reagent, here:
http://www.h2o2.com/industrial/fentons-reagent.aspx?pid=143&...
As a surprisingly simple alternative to Fenton's, I found a patent detailing the use of SO2 or SeO2 and H2O2, without the need for any sort of
co-catalyst and using n-propyl acetate as a solvent:
http://www.freepatentsonline.com/5026925.pdf
Interestingly enough, they don't report any m-dihydroxybeneze as a result of this method. Unfortunately, most of their examples do use >70% H2O2
solutions.
Oh, and no, I have not personally tried any of these reactions, so I cannot offer specific advice.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
RiP057
Harmless

Posts: 29
Registered: 26-10-2010
Member Is Offline
Mood: No Mood
|
|
seriously that is an enormous amount of work for next to nothing.... maybe fun but purchase... there are a million different sources.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Is there some alternative to fenton's reagent that doesn't use h2o2?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Fenton's depends on the OH radical, and peroxides are the common/easiest way to get that. As the pH is slightly acid, various peroxide salts might be
usable - don't know for certain.
There's not much else that does a decent job, the OH radical is a very strong oxidiser. I believe Ce(IV) salts can give similar oxidations, but
without very decent yields. Most of the bacteria that chomp on aromatic rings use peroxide.
Try a bit of searching for aromatic ring oxidations.
[Edited on 14-12-2010 by not_important]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
There is also way to make salicylamide from phenol, urea and ZnO catalyst so then I could make catechol from salicylamide with steps written in the
first post of this thread. All without H2O2 
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Random, are you talking about this method: 10.1016/j.catcom.2007.11.006
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Yes, that is the reaction I am talking about.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Random  | There is also way to make salicylamide from phenol, urea and ZnO catalyst so then I could make catechol from salicylamide with steps written in the
first post of this thread. All without H2O2  |
Did you note the conditions for such reactions?

205C, 12 hours, 16% yield.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
Another table, this time from 10.1016/j.catcom.2007.11.006 :
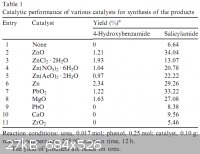
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
A note on using. Li and alcohol.
Your professor never tried this or
We would have used it for claisen
Years ago.
It makes hydroxides not alkoxides the
Electrons are held too tight.
Plus I tried many times.
e3500 console login: root
bash-2.05#
|
|
|
atomicfire
Harmless

Posts: 37
Registered: 7-2-2011
Member Is Offline
Mood: shaprening my molecular scissors
|
|
Sorry for digging this up, but I was looking at synthesizing catechol from phenol and have come across many varying methods. Has anyone found a
simple yet effective treatment of phenol to catechol?
I would like to try the direct hydroxylation of phenol via hydrogen peroxide but what type of catalyst would I need? I have seen the use of many
exotic ones, but what is the reason?
ban DHMO
|
|
|
dean stark
Harmless

Posts: 12
Registered: 17-3-2011
Member Is Offline
Mood: No Mood
|
|
Aspirin
Okay, so not terribly straightforward but...
Aspirin to 3-acetylsalicylic acid via ortho-Fries arrangement*, followed by decarboxylation and Dakin oxidation, not necessarily in that order.
In this* paper (which I don't have electronically), they perform ortho-Fries rearrangement on 2-acetoxynitrobenzene by microwaving for a few minutes
with zinc dust in DMF.
Seems the hardest part would be decarboxylation. Would simple heating work or would it require something like Quinoline?
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
The Fries rearrangement adds a hydroxyl group to the ring, and attaches the alkyl part of the ester directly to the ring (in the 2 or 4 position
apparently). So the closest you could get to 3-acetylsalicylic acid would be maybe 2-hydroxybenzaldehyde, but that would be if you *started* with the
methyl ester of phenol, not aspirin. Fries rearrangement on aspirin (if it even worked, which I doubt) would give you a triply substituted ring and
leave you no closer to catechol than before.
Decarboxylating salicylic acid can apparently be done via simple heating, but I haven't had any success doing it preparatively at temperatures up to
195C (including attempts with copper catalyst). Probably not hot enough, may need something like 230C to proceed at a reasonable rate. Anyway if you
did that you would have phenol, which as some others have suggested might be a route to catechol.
There is a patent out there somewhere on the oxidative (via air) decarboxylation of aromatics using copper catalysts; in that process the carboxyl
group ends up replaced by a hydroxyl one. So if this worked on salicylic acid, it would provide a direct route to catechol. However as I recall their
process didn't work well at all when there was an ortho-hydroxy substituent, so it's not really applicable here.
|
|
|
dean stark
Harmless

Posts: 12
Registered: 17-3-2011
Member Is Offline
Mood: No Mood
|
|
Pretty sure the ortho-Fries rearrangement of Aspirin IS 3-acetylsalicylic acid (unless I'm getting the numbering wrong, and BTW I'm using acetyl in
the sense of acetylbenzene which is acetophenone...).
The Fries rearrangement doesn't ADD a hydroxyl group - it's already there. From Wikipedia: The Fries rearrangement ... is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of lewis
acids.
So the acid part of the ester, which is acetic in this case, migrates to the aromatic nucleus.
More details and diagrams from the paper are available from the Organic Chemistry Portal.
|
|
|
| Pages:
1
2
3
4
5 |