| Pages:
1
2 |
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Dornier, don't you want to try the same with KClO4 instead of KClO3, maybe even more powerful?
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I would, but I don't own any perchlorates at all. I also doubt it would be more powerful, as its melting point is higher than most fuels' autoignition
temperatures. Anyone here who is able to test this with KClO4?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I have some perchloate. However I dont own any benzoate. Have you tried nano hexamine mixtures? problem is that they dont melt, however I can try
dissolving hexamine and ammonium perchlorate in water and recrystallizing them, it should be very well mixed then. Potassium perchlorate is too
insoluable, sodium and lithium perchlorate forms hydrates.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Do you have any fuel normally used in whistle mixtures? Hexamine will not work here, it should be an aromatic salt.
Bert, I tried copper benzoate with KClO3 in a 20/80 ratio by weight. It's a beautiful light blue mixture, which
deflagrates quickly with a blueish flame. It ignites before melting, but strong heating and confinement caused detonation.

Have you tested Ba(ClO3)2 and potassium benzoate yet?
EDIT:
I pressed the remaining 0.1 g in a thin walled aluminium foil tube, again with a few mg KClO3/K3[Fe(CN)6] as primer.
It went of with an amazingly loud and sharp bang and shattered the aluminium plate it was placed on. So yet another mixture with potential as primary.
[Edited on 9-3-2014 by Dornier 335A]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Outside of high pressure (rocket engine) use, NH3ClO4 often isn't the oxidizer of choice for speed.
NH3ClO4 fireworks star mixtures are generally harder to ignite, slower burning and have lower critical speeds than similar
mixes made with KClO4.
For uses where the solid reaction products left by KClO4 detract from flame color & transparency, NH3ClO4 is
often substitutes- But almost always with a few % of the Potassium salt to enhance ignition and burn speed.
KClO4 and hexamine mixture burns slowly compared to benzoate or salicylate fuels. We sometimes substitute a few % hexamine for other hot,
fast burning fuels in fireworks star mixes to slow & cool the burn, as in blue mixes that would turn to green at too high temperatures.
Usually if you melt the perchlorate in a mix, the stuff is on fire...
(edit)
No, I have not yet tried to melt any whistle fuel analogues. Kind of busy with the work that pays the bills-
[Edited on 9-3-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The use of aromatic salts compounds is needed:
-1°) to make the salt resist the heating without sublimation or volatilisation
-2°) because of the high % of charing when heated so the mix is a kind of chlorate/C mix (with atomic or molecular C).
-3°) the oxydation/burning set free some organic acids that participates to the unstability of the chlorate and lower the activation energy during
the combustion process.
For this reasons also reaction with perchlorates or with hexamine will behave very differently and not go D2D in tiny amounts.
Maybe hexamine salt and chlorate will provide a different result, but this is another story because in the mix you would have an acidic moeity
(hexamine salts are mildly acidic) and so some HClO3 would form and fix to the hexamine to make hexamine di-chlorate salt... Most organic amine
chlorate salts are sensitive HE (choc, friction and heat) even more than the perchlorate ones. So this would be like a gun-powder composition with a
detonating stuff dispersed into it (between detonation and deflagration).
Edit:
-Copper doesn't stand well chlorate...I had some CuCl2 and CuOCl used to get some green shade decomposing into NaClO3 composition there was a Cl2
smell.
-For the stupid ones don't forget the rule no ammonium salt mixed with chlorates...so no ammonium benzoate mixing with KClO3 or NaClO3! Spontaneous
ignition might occure at any time.
[Edited on 10-3-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
packetforger
Harmless

Posts: 48
Registered: 21-2-2014
Member Is Offline
Mood: Condensing
|
|
As P. Zrealone mentions, the use of copper salts mixed with chlorates is in general a bad idea, copper contamination of chlorates is generally
percieved as a pretty awful safety risk... Cannot imagine doing it deliberately! It is probably for this reason - copper sensitizes chlorate quite a
bit, and the benzoate is easily enough going to act as a fuel...
As Bert mentioned, Ammonium Perchlorate is a not-so-great oxidizer for use in stuff outside of rocket propellants as it is fairly slow stuff. it would
actually be interesting though to test Ammonium Perchlorate recrystallized with hexamine as a rocket propellant though, I imagine said mix would
evolve a fair amount of gas and be a decent propellant, though it would likely need a bit of aluminium added to get it going fast enough to get stuff
off the ground. Using a deep core would be advised as opposed to an end-burning design.
These mixtures, while interesting, do not strike me as particularly suitable as replacements for primaries, mostly because all of them seem to suffer
some inherent instability (f.ex: copper and chlorate...). The chlorate and sodium benzoate mix does look interesting though, I would love to give it a
shot sometime if it seems to be able to self confine in such small amounts. Melting a small quantity of it onto a bridgewire might make a decent
electrically fired squib.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I ran a series of tests with sodium chlorate and sodium benzoate. At first the mixture seemed to behave identically with the potassium chlorate
version, with the exception that now the chlorate melted before the benzoate.
What became apparent after a few tests was that the mixture was more difficult to detonate properly than with KClO3. It did not always go
high order when confined in aluminium foil and heated. The aluminium fragments were also larger which indicates less brisance. I had expected the
opposite. The somewhat disappointing results are likely due to the melting point and decomposition point of NaClO3 not being matched very
well by those of the fuel.
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Perhaps this mixture offers a more convenient and reliable way of thermal initiation without the undue sensitivity that plagues most primaries.
Although the fact that it needs a rather controlled gradual heating to first melt and then detonate, will most probably complicate things. A bulky
metal or perhaps a ceramic interface that transfers the heat gradually from a pyrotechnical mixture to the KClO3-bezoate charge might work in this
case, but it will not be convenient and tends to become bulky, cumbersome and unpredictably delayed as most thermo-shock based initiators. Though as a
proof of concept test, it might be worth investigating....
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Dornier 335A  | I ran a series of tests with sodium chlorate and sodium benzoate. At first the mixture seemed to behave identically with the potassium chlorate
version, with the exception that now the chlorate melted before the benzoate.
What became apparent after a few tests was that the mixture was more difficult to detonate properly than with KClO3. It did not always go
high order when confined in aluminium foil and heated. The aluminium fragments were also larger which indicates less brisance. I had expected the
opposite. The somewhat disappointing results are likely due to the melting point and decomposition point of NaClO3 not being matched very
well by those of the fuel. |
Maybe NaClO3 cristallises with water or catch water from the air more than KClO3 does...are you sure the NaClO3 was anhydrous and kept away from moist
air?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hello everyone, been absent here awhile. This topic sparks my interest. As PHILOU Zrealone and
Dornier 335A concur, double replacement is forming a double salt. Co-crystallized salts containing oxidizer and fuel , ordinarily
tend to be sensitive. Chlorate with organic fuel always react to some extent. What is really remarkable is that this mixture is able to melt without
immediately exploding , has high explosion temperature and high impact insensitivity. The expected properties of the resultant double salt will
exhibit more susceptibility to shock given the intimate composition of anions, as Dornier 335A has already observed www.sciencemadness.org/talk/viewthread.php?tid=29259#pid3218...
Elsewhere I had proposed :
" Tricyanomethane and Trinitromethane form ionic metal salts. A way in which the two
moieties might be compounded is by a solution of equal parts of Magnesium Tricyano -
methanide and Magnesium Trinitromethanide co-crystallized as a double salt , thus
Mg{C(CN)3}2 + Mg{C(NO2)3}2 => 2 Mg{C(CN)3C(NO2)3} => 2 MgO + 10 CO + 6 N2
In the same way , Benzoate and Chlorate salts , both of either Calcium or Magnesium should result in a double salt. Of course this would be a compound
with a one Chlorate anion per Benzoate anion. Performance would be much reduced. A melt of Benzoate in excess Chlorate , of course yields better
oxygen balance. The wavering quality of the product produced must be attributable to inconsistent inhomogeneous crystallization. www.sciencemadness.org/talk/viewthread.php?tid=29259&pag...
A way to obtain a well uniform product is to first obtain the stoichiometric double salt and then subsequently to blend that in with a greater amount
of Chlorate salt.
__________________________________________
First we obtain the stoichiometric Calcium Benzoate Chlorate double salt
Ca(C7H5O2)2 + Ca(ClO3)2 => Ca2(C7H5O2)2(ClO3)2
Having a molar weight ratio of 282 : 207
Next we compound this with Sodium Chlorate
Ca2(C7H5O2)2(ClO3)2 + 3 Na(ClO3)
Having a molar weight ratio of 489 : 320
which will detonate into
=> CaO + CaCl2 + 3 NaCl + 14 CO + 4 H2O + H2
.
[Edited on 14-6-2014 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
The question of what are the optimal ratios of Benzoate to Chlorate confirms that zero oxygen balance yields the
highest heat value per weight of material.
Ignoring everything to the left of NaCl in the balanced equations seen below , and just summing the heat of formation of CO2 , CO , and H2O , in ratio
to the total weight of the ingredients , the higher the number , the higher the heat value , an additional 192 kilocal for every mol of NaClO3.
This does not automatically confer higher brisance. The number of gas mols is constant while the number of mols of solid products increases , and as a
result the gas proportion is reduced with better oxygen balance. ( High velocity explosives in composition with Aluminum trade some
brisance for enhanced blast. The optimal amount is around 18 to 20 percent which yields a third of detonation products as solid
alumina.) This also occurs for the stoichiometric mixture , with 8 mols NaClO3 , where best performance will likely be seen.
The following values in kilocalories per mol are applied for thermodynamic calculations
- 94 for CO2 , - 26.4 for CO , - 57.8 for H2O
Ca2(C7H5O2)2(ClO3)2 = mol wt. 489 , NaClO3 = mol wt. 106.5 . . . . . . . . . . . . . . . . . . . . . .
.∆H / mol wt. . . . . . . .solid mols / total of mols
Ca2(C7H5O2)2(ClO3)2 + 3 NaClO3 => CaO + CaCl2 + 3 NaCl + 14 CO + 4 H2O + H2 . . . . . . 601 / 809 . .~ 0.74 , . . . . % solids . .~ 21
Ca2(C7H5O2)2(ClO3)2 + 4 NaClO3 => CaO + CaCl2 + 4 NaCl + 2 CO2 + 12 CO + 5 H2O . . . 794 / 915 . . ~ 0.87 , . . . . % solids . .~ 24
Ca2(C7H5O2)2(ClO3)2 + 5 NaClO3 => CaO + CaCl2 + 5 NaCl + 5 CO2 + 9 CO + 5 H2O . . . 986 / 1022 . . ~ 0.96 , . . . . % solids . .~ 27
Ca2(C7H5O2)2(ClO3)2 + 6 NaClO3 => CaO + CaCl2 + 6 NaCl + 8 CO2 + 6 CO + 5 H2O . . .1199/ 1128 . . ~ 1.06 , . . . . % solids . .~ 30
Ca2(C7H5O2)2(ClO3)2 + 8 NaClO3 => CaO + CaCl2 + 8 NaCl + 14 CO2 + 5 H2O . . . . . .
. 1605 / 1341 . . ~ 1.2 , . . . . . % solids . .~ 34
12 : 21 parts by weight
Dividing the mol weight of the stoichiometric mixture 1341 by the mol weight of each of the preceding mixtures individually , you obtain a factor by
which to multiply it's ∆H value , thus normalizing the shown values for an equal weight of material ( 1341 ) in every case. This works out to 996
∆H for 3 mols NaClO3 , 1164 ∆H for 4 mols , 1293 ∆H for 5 mols , 1425 ∆H for 6 mols.
.
[Edited on 15-6-2014 by franklyn]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
new use for tetraphenylborates!
Naively I think the stability of the molten stuff is repulsion between fuel anion and oxydizer anion. In order to get a neutral intermediate, MO(-)
breaks off leaving either PhC=O (+) or the chloryl cation, or some radical. If that's true, perhaps the properties and stability could be fine tuned,
deliberately and science like. Patents cost and if it's obvious to me it's obvious.
Phthalate and terphthalate with two carboxylates: dead weight, but maybe less reactive?
Gallates used to be used in whistle composition, and salicylates. I'd guess those are worth investigating in the molten MClO3 or 4 mixture,
and common too.
If we want to get baroque there are the anisic acids, aminobenzoic, etc.
I doubt the arylsulfonates have [had any reason to] been tried in pyrotechnic compositions before. And if we want to be absurd, tetraphenylborate
salts are known.
[Edited on 15-6-2014 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Along the same lines I thought of this , mixing Calcium Chlorate and Calcium Picrate
to form a double salt with a much improved oxygen balance. Perchlorate would do
nicely also. Though it's been contemplated by some , melting picrate salts seems
risky , www.sciencemadness.org/talk/viewthread.php?tid=6551
Formation from cold solution seems the proper route.
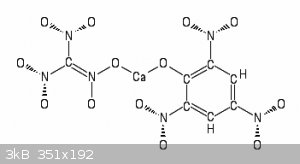
2 Ca(ClO3)2 + 2 Ca(C6H3N3O7)2 => 2 Ca(ClO3)2 (CaC6H3N3O7)2
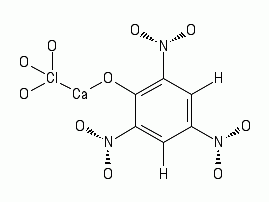
=> 2 CaO + 4 CO2 + 8 CO + 2 H2O + 3 N2 + Cl2
____________________________________________
While on this subject , another possible double salt using Calcium to link Trinitromethane with
Trinitrophenol.
=> CaO + 4 CO2 + 3 CO + H2O + 3N2
.
[Edited on 16-6-2014 by franklyn]
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
PHILOU Zrealone, the mixture ignites at more than 250°C. I'm sure there won't be any water left in the liquid.
I believe the goal is to find the best compromise between homogeneity of the mix, oxygen balance and actual usefulness. The molten mixture is
homogeneous and can have perfect oxygen balance (I mixed mine to -10%), although not very useful in practice due to the high melting point.
I was able to detonate melted and quickly solidified flakes of the mixture, but those are likely to be less homogeneous. What about cooling the liquid
down faster, like dripping it into liquid nitrogen?
Double salts' complete homogeneity even in the solid state is indeed interesting Franklyn. I think smaller or better oxygen balanced
anions are the way to go with chlorate, rather than mixing the double salt with excess chlorate afterwards. Using calcium as the cation might be
problematic because the benzoate crystallizes with water and the chlorate is very hygroscopic.
I will try a mixture (preferably molten or cast) of NaClO3 and calcium picrate as soon as possible, hopefully this week. My theoretical
calculations say it will have exceptionally high performance (>8000 m/s at max density). KClO3 or NaClO3 and potassium
picrate is also on my list.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Okay, I've synthesised three metal picrates now: potassium, calcium and sodium. I tried combinations with sodium and potassium chlorate. Below you can
see melting points and ignition points for the ingredients:

Data from Urbanski, Fedoroff and Wikipedia
The criteria for a mixture like KClO3/sodium benzoate seem to be the following:
- At least one of the components have to be molten at the ignition point.
- The fuel and oxidizer have to be compatible in the melt.
- The oxidizer should not decompose first, otherwise bubbles will form.
- And finally the mixture should detonate fairly easily.
Only one of the six mixtures fulfilled all four criteria and gave satisfactory results. It was potassium chlorate and sodium picrate, 50/50 by
weight. One milligram confined in aluminium foil and heated briefly in a flame exploded with incredible power, turning the foil into hundreds of
fragments. Potassium chlorate and potassium picrate ignited before melting. Sodium chlorate and potassium picrate started to bubble and detonated very
seldom. Calcium picrate behaved very strangely. It deflagrated only slowly, even though Fedoroff wrote it "explodes violently at 323-28°". A mixture
with potassium chlorate could detonate if confined well enough and heated quickly, but its brisance was not very impressive.
One mixture which could work is sodium chlorate and copper picrate; copper picrate is said to explode violently at 282-287°C. (I know copper/chlorate
is generally a bad idea but it's worth a try.)
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
copper and chlorate? you sure this isnt because it could by accident get near ammonia and form the primary explosive tetraammine? when i get all the
acid out of my copper chloride ill attempt to make copper chlorate, i recall it has even been sold in smaller amounts for use in exotic fireworks
and if pyrotechnicians dares to handle it, then there shouldnt be that much worries about it
but again, you cant get smarter by assuming you know it all
|
|
|
jjgoh
Harmless

Posts: 24
Registered: 24-12-2013
Member Is Offline
Mood: No Mood
|
|
This type of composition is very powerful when melted and it does explode but not deflagaration.
I Wonder can I twist the formula in order to make it explode in no time when a fire near to iit.
Currently using this formula :
Potassium Chlorate = 7.11%
Sodium benzoate = 2.99%
.
KClO3 is laboratory grade where sodium benzoate is food grade which is obtain from a bakery shop
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
What is the other 90%, or did you make a mistake?
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
| Pages:
1
2 |