| Pages:
1
2
3
4 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
And precipitated copper is somewhat brighter ... at least as long as one uses pure reagents! (i.e. no citrus flavours etc  ) )
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
<snip>
Apart from that... I rubbed the powder onto a surface, and it actually became shiney, <snip> |
Chemoleo, does the surface become conductive?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well, as I said above, I noticed a massive increase in conductivity, if the Cu powder was present. So yes, it is. However, the oxide layer that forms
after a few minutes/hours on air prevents conductivity. That is why the powder that I left on air to dry didn't conduct. Only the freshly
HCl-washed powder conducted (which was still slightly wet with distilled H2O, while the very same distilled H2O had a conductivity up to 500 kOhm).
That's why I recommended to keep the powder wet (i.e. in EtOH) until before use, and then to dry it rapidly.
Or, use the PVP from the chemical abstract paper above to protect it (it's mentioned to be employed as a protectant).
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
As I had never made copper powder in any way I thought I'd give it a try.
I took ~80ml of strong CuCl2 solution (I don't know how strong it was as I was evaporating it down to get the CuCl2) and added few pieces of
Al-foil. It boiled vigorously and some dark red powder witch was mostly very fluffy formed on the bottom. I then took a piece of pure Al-bar ( ~40cm x
5cm x 0.4cm) and supported it so that it would sink into the solution as it dissolves.
This method as I noted does not make very fine powder. But it's ok until I get some ascorbic acid as I think I’ll clean this batch with some
HCl and close it into a glass vial so it will remain nice and shiny in my shelf.

|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
Would formic acid perhaps prove sufficient for reduction? just for the reason that would entail no extra expense to purchase ascorbid acid, as I have
a bottle ofr 40% formic lying around much untouched
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Yes, formic acid will do.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Now, theoretic, where did you get that idea from ? Although formic acid has the HC=O group, it doesn't mean it has as strong a reducing character
as ascorbic acid.
You may know the Fehling test, where aldehyde sugars are employed to produce Cu2O. Note, Cu2O. So why would formic acid suddenly produce Cu?
Obviously the reduction with ascorbic acid isn't really obvious, else these fellows wouldn't have deemed it worth publishing in 2003. Not
any reducing group does. It looks like ascorbic acid is quite unique in this ability to reduce Cu2+ to Cu!
My guess is nothing will happen at all, if you are lucky you might get Cu-formiate.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Rex- Not with CuSO4. This anhydrous salt can be used to dry HCOOH. chemoleo is of course correct.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Experiment:
Formic acid does NOT work.
Hydroquinone does NOT work.
Sodium Bissulfite produces a precipitate that's NOT Cu.
Ascorbic acid WORKS. It takes a bit long though. Some copper deposits on the glass surface (possible mirror?) after 1/2 hour. Overnight a deposit
forms.
A thought: I have doubts if this is indeed much more fine than the powder created by Zn powder in CuSO4. The vit C powder precipitates quickly, as
chemoleo pointed out. The copper powders I have made from Zn sometimes take long to precipitate. Maybe the crystals growing on Zn are more
"fractal" than the ones reduced by vit C.
But I haven't done any tests to back me up on this, just an impression.
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
I tried the precipitation of Cu using Ascorbic acid and CuSO4. The Cu ppt formed is very fine, but nearly as fine as that which is precipitated by the
rxn between Zn ad CuSO4. Could the size of the particles of Cu depend on the concentrations of the solutions being used? I used a somewhat dilute
soln. of Ascorbic acid.
Theory guides, experiment decides.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Well, I went out and bought the purest vit C I could find OTC. The contaminants are methylcellulose, microcrystalline cellulose, talc, magnesium
stearate.
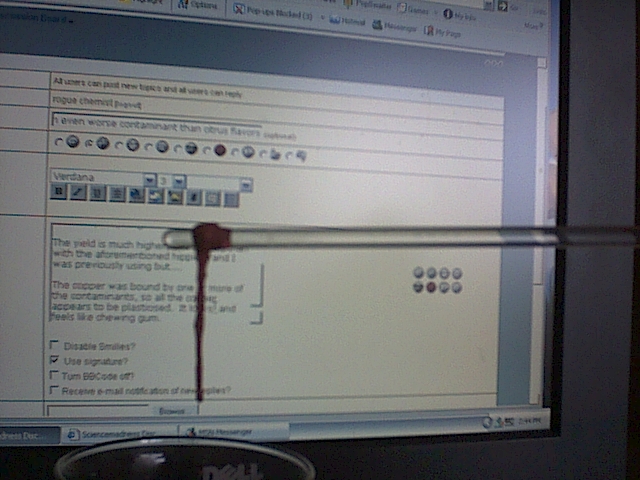
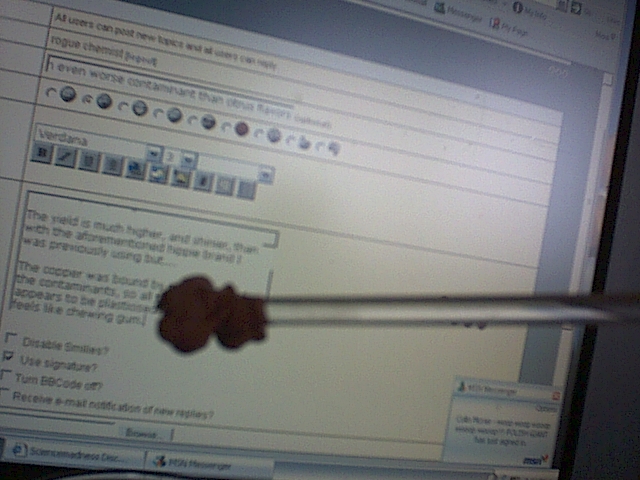
The yield is much higher, and shinier, than with the aforementioned hippie brand I was previously using but....
The copper was bound by one or more of the contaminants, so all the copper appears to be plasticised. It looks, and feels like chewing gum.
Sorry, for the crappy pics, my digital camera is very bad. In the pictures the copper is actually brighter than it appears.
EDIT: I am just going to order some ascorbic acid instead of dealing with these contaminants
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Sorry about the double post, just wanted to show another picture.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Rogue - why don't you use lemon juice? It might be cleaner than the stuff you are working with!!
Anyway - on the degree of fineness- tacho/esplosivo you may have noticed that there is very fine stuff, and the more grainy variant. The more grainy
variant can, when dry, be crushed, to be very fine. This is when I got a piece of paper shiny with copper dust 
Also - I think the overall surface area of the precipitated powder has to be greater by definition than Zn-reduced Cu, simply because reduction is not
localised in space to the reductant particles (i.e. Zn). Instead, reduction occurs everwhere at once, i.e. in solution.
What I rather suspect is that the Cu conglommerates, aggregates or whatever, in a loose kind of fashion. This, as it seems from the Chemical
Abstracts, above, can be prevented with various agents, here a polymer. Might be a nice idea to do this whole thing with polyethyleneglycol, or even
glycerol, to see whether the coarsity of the Cu changes. However, here the hydroxyls might interfere.
Hell, I might give it a go tomorrow 
Edit: Rogue, the picture is above 100 kb, so it might be deleted. But I don't know whether that's true for pictures in the upload directory
only.
[Edited on 18-10-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 206
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
What about using dissolved dextrin to create a smaller precipitate? It works for lead azide, why not here?
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
<snip>
Also - I think the overall surface area of the precipitated powder has to be greater by definition than Zn-reduced Cu, simply because reduction is not
localised in space to the reductant particles (i.e. Zn). Instead, reduction occurs everwhere at once, i.e. in solution.
<snip>
|
chemoleo,
Your work is great, as the document published only in 2003 proves. It may be a very specific property of vit C to reduce copper like that.
However, just for the sake of discussion, I disagree with your statement above. It's about crystal growth. They may grow on the Zn surface or on
nucleating points (!?). One doesn't have to be larger by definition.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
A solid dispersion can NEVER achieve the "surface area" and reactivity of a dissolved agent.
With vitamine C you are working with single molecules of vitamine C, whilst the Zn powder consists of clusters of more than thousand atoms with
relatively little surface.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
The fact is that the precipitated copper is not in a colloidal state. Its a powder, so we are talking of crystal growth on a nucleating point.
Wait!... what am I doing? BlahBlahBlahing! I'm sorry Descartes!
I'll experiment tonight with Zn powder and VitC and measure time of precipitation.
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
I thought the realy active stuff was made by amalgamating the metal with aluminium and then treating it with alkali.
I have used Raney nickel but there is also Raney cobalt and Raney copper
mick
Edite typo
[Edited on 18-10-2004 by mick]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Tacho, I should think that we are dealing with amorpheus aggregates of Cu here, rather than 'crysallised' Cu. I.e. one Cu atom binds
randomly to another, in an unordered fashion. This explains why the powder can be crushed easily. While, when it is derived from Zn powder, the
particles will at least have the size of the Zn powder, whereby the reaction allows for better alignment of the Cu atoms - so if anything - Zn-derived
Cu is more crystalline than Cu precipitated straight from solution.
Anyway, I fully agree with vulture on this.
Nice idea about the dextrin btw. Shame I haven't got any here 
Mick, of course one can make Cu that way too - but this is bound to be not quite as simple as with Zn/Vit C. But ultimately that should yield
extremely fine metal powder.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
| Quote: |
I have used Raney nickel but there is also Raney cobalt and Raney copper
mick
|
No worry mate, we'll through another shrimp on the barbie!
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 206
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
Chemoleo,
Do you have any corn starch in your kitchen? You can turn it into dextrin fairly easily by baking in an oven @425F until golden brown.
[Edited on 19-10-2004 by FrankRizzo]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Indeed. I turned 500g of corn starch into dextring by keeping it at 200 degrees C for 2 hours
EDIT: Or I was drunk when I did that: I feel... uncertain.
[Edited on 2004-10-19 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
The vit C copper is much better looking than tha Zn copper. No matter how much you wash the Zn copper with HCl or H2SO4. BTW, concentrated HCl
(fuming) made both coppers disappear (without fizzing->no zinc)! This surprised me.
The vit C copper looks like copper. The Zn copper looks like grey-redish dirt.
The vit C copper seems to have coarser grain and finer grain, I mean it's "graduated" from fine to coarse. The finer grain is quite
finer than the one obtained by Zn, it remains "floating" long after the Zn copper has settled. The coarser grains seems to be still finer
than Zn grain, but not much.
Chemoleo and Vulture:
My reasoning was based on the silver crystals that grow on a piece of copper in silver nitrate solution (the classic "silver tree"
demonstration). They are quite smaller than the copper piece. A similar mechanism could produce smaller copper crystals than the Zn grain. That
doen't seem to be the case though. Maybe, as chemoleo pointed out, they are amorphous.
Anyway, I'm very interested in this. This copper powder seems quite different from the ones I have seen (from Zn and Al). I hope I can find a
cheap source of rather pure ascorbic acid to do some further testing. It looks so conductive and reactive... You should try to make some, axehandle,
I'm sure it will make your brain start ticking.
Chemoleo, what was the reasoning behind you choosing ascorbic acid as a reducing agent? Maybe we could find another one.
Dextrin huh?
[Edited on 19-10-2004 by Tacho]
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Copper precipitated on steelwool, the kind I posted a pic in the benzene-thread looks very nice, copperlike. It forms these "trees" which
can easily brushed up and yield finely divided copper of the color it should look like.
Dont forget to add some NaCl to get this going.
Copperoxide can be reduced to elemental copper by hot ammonia vapors .
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
| Quote: | Originally posted by Tacho
concentrated HCl (fuming) made both coppers disappear (without fizzing->no zinc)! This surprised me. |
Copper shouldn't be attacked by HCl, as copper is lower on the reactivity series than hydrogen. Could there have been an oxidizer in your acid?
On the topic of acquisioton of vit C, I've found it in a vitamin store, but I can't seem to firgure out its purity. 8oz has a strength of
1065g??? But 8oz = 226.8g. Does anyone know what to make of this?
edit: The link function doesn't seem to eb working right, so here's the address:
http://www.vitaminshoppe.com/browse/sku_detail.jhtml;$sessio...
[Edited on 19-10-2004 by neutrino]
|
|
|
| Pages:
1
2
3
4 |