| Pages:
1
2 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What I found most interesting about the patent is that it provides a way of controlled crystallization of the usual double salt , by varying the
concentration .
Any residual solution may be concentrated
and reused .
The description in PATR indicates that the
double salt is formed in nearly 100% yield
and is a quite good initiator , not light sensitive nor unduly friction or impact sensitve , storage stable at normal temperatures , but responsive to
flame .
Initiating properties compare favorably
with MF and LA . The technical difficulty
for manufacture is low , so the only negative seems to be the cost of the silver , and the double salt is otherwise
a good initiator if the reported data is correct .
A thought I have is that it seems possible
that the methyl acetylene is possibly being
cleaved and the product is actually the same as the usual double salt from ordinary acetylene . This could be determined by comaparing the properties
and weight and solubility of the product
with the properties of the usual double salt . Even if it is the same double salt ,
it would still be very useful if MAPP gas
could substitute for regular acetylene .
Another interesting detail mentioned in the patent is that a small amount of
Fe(NO3)3 in the solution prevents any precipitation of free silver which should increase the purity of the product .
[Edited on 2-10-2004 by Rosco Bodine]
|
|
|
Axt
National Hazard
   
Posts: 798
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Igniting a larger amount of the copper product up today resulted in a self sustaining combustion, smoldered as the black ash fell away from it. I take
this as being good evidence that it has indeed formed a chloride complex and not some sort of basic chloride. But the colour, a very bright
fluorescent green doesnt match the chloride complex of acetyline, Cu2C2.CuCl.H2O, which is dark violet (PATR).
Conclusion, I think it is a methyl acetylide, and it will form complexes. But the evidence could also be explained by a non-explosive CuCCCH3, as
noone knows what its properties are, or cleaving & forming higher chloride complexes of Cu2C2, as we dont know what they look like.
<center><img src="http://ww1.altlist.com/~58717/pulse.altlist.com/images/chloridecomplex.jpg"></center>
[Edited on 3-10-2004 by Axt]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Possible evidence for existance of multiple forms of SMA, possibly double salts
I have prepared SMA twice since this thread started. Both batches were made within 2 days of each other. As mentioned on the first page of this
thread, I prepared my SMA by dissolving 2g of silver in 2.5mL of nitric acid, adding water to increase the volume to 40-50mL, then bubbling propyne
through.
The first batch the propyne was only bubbled through for under five minuits, where as the second batch the propyne was bubbled through for around
20min.
Well, here is the evidence, The SMA from both batches has been lying around for a couple weeks. The SMA from the first batch has now turned a dark
grey where as the SMA from the second batch has only turned slightly light grey, only off-white. Both samples still deflagrate identically with the
same appearance as when fresh. I am assuming that the different colour changes indicate different photosensitivities, and therefore are different
compounds.
Thoughts?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well, if you think there' s a double salt with AgNO3 in one case but not the other, you could always take each and analyse it for the presence of
the nitrate ion.
I am sure there are specific tests for it, isn't the Na-prussid a specific one?
Anyway - this might be a way to go about it.
(i.e. you could subject a certain amount of each batch to NaOH, to precipitate the Ag as Ag2O (i guess), while then analysing the remaining solution
for NO3-)
[Edited on 15-10-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Although it will explode from a hammer blow (but less sensitive than Ag2C2.AgNO3), silver methylacetylide (C3H3Ag) is a feeble explosive. This feeble
property is not really redeemed when it is mixed with several strong oxidizing agents (like KClO3 or KMnO4). Beilstein says the decomposition
temperature is about 150 deg. It can also be destroyed like silver acetylide, by forming a water suspension of it and then adding concentrated HCl,
which will release the alkyne gas.
|
|
|
Axt
National Hazard
   
Posts: 798
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Even though the Ag salt is quite a weak explosive, resembles blackpowder in its effect. I'd be interested if anyone can form the Cu salt of MA. Though
I've failed in my attempts, it can be done. I searched for preparations but all are less then convenient (though they also give long winded routes to
the Ag salts).
<b>Copper and silver ethynyl co.ovrddot.ordination complexes</b>. Blake, D.; Calvin, G.; Coates, G. E. Univ. Durham, UK. Proc.
Chem. Soc. (1959), 396-7. http://dx.doi.org/10.1039/PS9590000377
Abstract
The Cu and Ag salts of substituted acetylenes are considered to be co.ovrddot.ordination polymers in which there is substantial back
co.ovrddot.ordination from filled d orbitals of the metal to at least 2 acetylene groups. It was found that, although CuC2Me is insol. in N bases, it
is readily sol. in a toluene soln. of Et3P to form yellow, cryst. Et3PCuC2Me. In addn., the complexes Et3PCuC2Ph, m.p. 138.5-139.5; Et3PAgC2Ph, m.p.
77.5-78.5; and Et3As-AgC2Ph, m.p. 89, were prepd. from the phenylethynyl salts. AgC2Ph is sol. in various amines. The degree of assocn. of
Et3PCuC2Ph is 3 in PhNO2 and between 3.3 and 3.8 in C6H6, while that of the corresponding Ag complex is 2 in PhNO2 and 2.6-2.7 in C6H6.
<b>Alkynyl compounds of transition metals. V. Alkynyl complexes with copper</b>. Nast, Reinhard; Pfab, Wolfgang. Tech. Hochschule,
Munich, Germany. Chemische Berichte (1956), 89 415-21. CODEN: CHBEAM ISSN: 0009-2940. Journal language unavailable. CAN 50:77632 AN
1956:77632 CAPLUS
Abstract
cf. C.A. 50, 9200c. When alkali salts of acetylenes in liquid NH3 are treated with suitable Cu compds. alkynylocuprates of the type M2[Cu(CCR)3] and
M[Cu(CCR)2] are obtained. Adding 2.07 g. CuI in liquid NH3 to 2.54 g. KCCMe in 80 cc. NH3, filtering off the ppt., and washing it with NH3 give the
ammoniate of K2[Cu(CCMe)3] (I) which, kept 2 hrs. in a high vacuum, loses the NH3 to give I. K2[Cu(CCH)3] (II) is prepd. similarly in 75% yield. A
magnetic measurement in a high vacuum using the cylinder method gives a susceptibility of Xg = -0.7 10-6 for II. "Boiling" 2.94 g. KCCPh with 1.5 g.
CuCCPh (III) in 130 cc. NH3 0.5 hr., filtering off the soln., concg. it to 80 cc., and cooling it 2 hrs. at -78 give 45% K2[Cu(CCPh)3].2NH3, cm.-long
pale yellow needles, losing NH3 in a high vacuum at 20 in 3 hrs. Boiling 0.4 g. KCCMe and 0.6 g. CuCCMe in 100 cc. NH3 0.5 hr. gives 100%
K[Cu(CCMe)2]. III (7.29 g.) and 5.08 g. NaCCPh in 400 cc. NH3 give 25% Na[Cu(CCPh)2].2NH3 (IV), long needles. Treating 6 g. IV in 60 cc. NH3 with
4.2 g. [Ni(NH3)6](SCN)2 in 60 cc. NH3 and cooling the violet soln. 1 hr. to -75 give [Ni(NH3)6][Cu(CPh)2]2.2NH3 which, 3 hrs. in a high vacuum,
changes to a black C6H6-sol. compd. (60%) of the compn. NiCu2(CCPh)4.4NH3. Treating 0.58 g. IV in 70 cc. NH3 with 0.37 g. CuI in 70 cc. NH3 gives 80%
CuCCPh.NH3, also formed from 1.23 g. CuI and 2.2 cc. HCCPh in NH3. Treating 2.16 g. CuI in 60 cc. NH3 at -75 with 0.71 g. NaCCMe in 100 cc. NH3 gives
80% CuCCMe, orange powder. Treating 0.486 g. KCCH in 80 cc. NH3 with 0.97 g. [Cu(NH3)4](NO3)2 (V) in 25 cc. NH3, distg. the NH3, taking up the
residue the next day with 150 cc. NH3, and evapg. the filtered soln. give 99% black CuC2, highly explosive. Adding slowly 1.84 g. V in NH3 to 1.12 g.
KCCMe in 100 cc. NH3, evapg. the NH3, and subliming the residue in a high vacuum at 20 give CHCCCH, m. 64.5, and 0.714 g. yellow CuCCMe. Boiling 5
min. a mixt. of 3.05 g. V and 1.75 cc. (CH2NH2)2 in 60 cc. NH3 and 2.79 g.
NaCCPh in 50 cc. NH3 and filtering the ppt. give PhCCCCPh (VI), m. 87; evapg. the mother liquor to 50 cc. and cooling it to -78 give
[Cu(H2NCH2CH2NH2)2][Cu(CCPh)2]2.12NH3, violet crystals which, in a high vacuum at 20, lose NH3 with the formation of the green-yellow
[Cu(H2NCH2CH2NH2)2] [Cu(CCPh)2]2.2NH3; on further standing in a high vacuum it loses (CH2NH2)2 and NH3 with the formation of VI and III.
<b>Oxidation of copper(I), silver(I), and mercury(II) methylacetylides in aqueous solutions</b>. Hoan, Huynh Manh; Brailovskii, S. M.;
Temkin, O. N. Mosk. Gos. Akad. Tonkoi Khim. Tekhnol., Moscow, Russia. Kinetika i Kataliz (1994), 35(6), 889-92. Publisher: MAIK Nauka,
CODEN: KNKTA4 ISSN: 0453-8811. Journal written in Russian.
Abstract
The primary product from oxidn. of Cu(I) and Ag(I) methylacetylides with copper halides is a dialkyne (MeCCCCMe), whereas the Hg(II) deriv. gives a
haloalkyne. Reactivities of various oxidants in reactions with Cu(I) or Ag(I) methylacetylides are reported. The mechanism is discussed for
oxidative conversions of metal ethynyl complexes.
<b>Polyacetylenes</b>. Schlubach, Hans Heinrich; Wolf, Viktor; Justus, Walter; Kohncke, Carl Heinz. Univ., Hamburg, Germany.
Justus Liebigs Annalen der Chemie (1950), 568 141-59.
Abstract
In attempting to prep. CuCCH by treating H2O satd. at 0 with C2H2 with 500 cc. H2O contg. 10 g. CuSO4, 40 cc. NH4OH, and 30 g. HONH2.HCl, the sole
product was Cu2C2 (which was also obtained whenever the conditions were varied); the mono-Cu deriv. was never isolated. In an expt., described in
detail, NaCCH (I) (prepd. from 8 g. Na) in 300 cc. liquid NH3 and 2.5 g. Fe2O3 treated 4 hrs. with a rapid stream of O gave 0.45 g. (HCC)2, isolated
as the hexabromide (II) (cf. Straus and Kollek, C.A. 21, 50). To I (from 2 g. Na) in 250 cc. NH3 at -40, 8.22 g. KMnO4 was added gradually, followed
by 11.2 g. dry NH4Cl and, with vigorous stirring, 22.4 g. Mn(NO3)2; the soln. was then treated with O as described above, yielding 35.35% II. The
accompanying, regenerated C2H2 (isolated as Cu2C2) was 46.5% (based on I used). The black residue (0.4 g.) in the reaction vessel failed to burn in
the Bunsen flame. In this reaction, 9.04 g. NH2OH.HCl could be used to replace Mn(NO3)2. At -70 under dry N, I (from 20 g. Na) in NH3 was shaken 2
days with 23 g. CH2Cl2 at about 23 (under 10-12 atm. pressure), the reaction vessel opened at a low temp., the NH3 evapd. at room temp., and the
cooled mixt. shaken 10 min. with 150 cc. PhCH2OH, cooled, and treated with 125 cc. 80% AcOH, then with 50 cc. H2O, and distd. in vacuo; the distillate
(collected at -70), treated with CaCl2 and redistd., gave 8% (1.4 g.) MeCCCCH (III), b. 75-5.5, m. -45 to -35, nD20 1.4717 (also given as 1.4817),
d204 0.7926 (also given as 0.7909). By a modification of this procedure in which NH4Cl was added in the later stages of the reaction, the yield of
crude III was increased to 23.6% (but almost 1/2 of this was lost in purification). BrCH2CCH (IV) (17.7 g.) added dropwise to I (from 10 g. Na) in
NH3, followed by addn. (after 5.5 hrs.) of 26 g. NH4Cl at -34, gave 16.3% III, or, when 90 cc. PhCH2OH and 30 g. AcOH were used in place of NH4Cl,
17.9% III (based on IV used).
By-products of this reaction were 44% of a Br-free polymer and 35% CHCCH2NH2. The following derivs. of III were prepd.: C5H3Cu, dark yellow,
exploding under shock or when rubbed; C5H3Ag, yellowish-brown, highly explosive, even when treated with concd. HNO3 (formed from aq. AgNO3 in NH4OH);
AgC5H3.AgNO3 (formed from AgNO3 and III in alc.). III with Adams catalyst in EtOH added somewhat less then 8 atoms H, forming C5H12, indicating,
however, that partial polymerization had also occurred. Br in CHCl3 with 2 g. III formed C5H4Br4, colorless oil, nD 1.555. Freshly distd. III (3.2
g.) in 4 vols. Et2O added to EtMgBr (from 15.9 g. EtBr) at -50 and treated gradually with dry CO2, followed by treatment at -15 with ice and satd. aq.
NaHSO4, extn. with Et2O, drying of the ext. with CaCl2, and treatment with EtOH-NH3 gave a reddish NH4 salt, which, when decompd. with 20% H2SO4 and
extd. with Et2O and petr. ether, gave 0.52 g. of a monocarboxylic acid, C6H4O2.H2O, needles (rapidly darkening at 35) and losing H2O only with the
greatest difficulty. III, treated with NaNH2 in NH3 at 60 and with MeI at -65, yielded (CCMe)2 (V), m. 64.5. The reaction leading to V gave exptl.
evidence that III was 1,3- and not a 1,4-pentadiyne. Evidently "methyldiacetylene" prepd. by Pr.acte.evost (C.A. 20, 2146) by the action of KOH in
alc. on MeCHBrCHBrCHBrCH2Br was not identical with III. MeCCH was prepd. in quantity as the Cu compd. (VI) by Nov.acte.ak's method (C.A. 5, 39) from
Mg2C3, which was formed by heating Mg in (O-free) H with an appropriate hydrocarbon at 700. The best yields (50%) of VI were obtained with C2H4;
C3H7, paraffin oil, and petr. ether gave 34-42% VI, whereas PhMe failed to react. VI (50 g.) shaken 2 days with O, followed by acidification with aq.
H2SO4, gave 14.1 g. (crude) V, purified by steam distn., m. 68.5. I was treated with (CH2Br)2 in NH3 at -60 and shaken 1.5 days at 20; after shaking
0.5 hr.
with NH4Cl, removal of the NH3, evacuation of the reaction vessel, heating at 100, neutralization of the resulting distillate with aq. H2SO4,
treatment with CaCl2, and redistn. in vacuo, there was formed 2.1 g. (HCCCH2)2 (VII), b760 84-9. (In the presence of PhCH2OH and AcOH this yield
decreased to 1.3 g.) The reaction temp. appears to be critical, because at 40, only polymerization products were obtained. I in NH3 reacting 10 hrs.
with 100 g. liquid CH2.CH2.O at -34, followed by evapn. at 40, treatment with abs. alc. under N, and, after 12 hrs., acidification below 50 with dry
HCl (with dimethyl yellow as indicator), filtration, neutralization with dry Na2CO3, and fractionation gave about 52% HCCCH2CH2OH, b760, 128.5-30,
which, with PBr3 and pyridine at -30, gave about 32% HCCCH2CH2Br (VIII), b760 109-10. By the usual condensation method I (from 8.2 g. Na) and 14 g.
VIII gave 0.4 g. VII. In the presence of 1 kg. Cu2Cl2, 400 g. NH4Cl, 100 g. Cu powder, 420 cc. H2O, and 24 cc. HCl within 5 days, 70 g. C2H2 reacted
with 60 g. MeCCH, giving, after distn. and fractionation in the presence of p-C6H4(OH)2, 6 g. pure MeCCCH:CH2, b. 59.2-60.1, nD20 1.4494 (cf. Jacobson
and Carothers, C.A. 27, 2419).
|
|
|
Axt
National Hazard
   
Posts: 798
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The attachment contains the preparation of the mercury "salt" of methylacetylene. Its not a salt rather an organo-mercury compund. Its precipitated
from K2HgI4, which is formed by mixing HgCl2, KI and NaOH.
Theres no mention of it being explosive, rather "After a crystallization from methyl alcohol the pure dipropinyl-mercury
melted at 203-204' (uncorr.) with slight darkening."
[Edited on 14-5-2008 by Axt]
Attachment: mercury methylacetylide.pdf (565kB)
This file has been downloaded 1013 times
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
Regarding synthesis of methylacetylene-propadiene from acetone, see http://www.freepatentsonline.com/4301319.html
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Axt
Even though the Ag salt is quite a weak explosive, resembles blackpowder in its effect. I'd be interested if anyone can form the Cu salt of MA. Though
I've failed in my attempts, it can be done. I searched for preparations but all are less then convenient (though they also give long winded routes to
the Ag salts). |
I've even tried ammonicial Cu(NO3)2. But below we'll know why no precipitate formed. Beilstein says hydrocarbons of the type C(n)H2(n)-2, which have
two carbon atoms in a triple bond react with aqueous or even acidic solutions of HgCl2, HgSO4, or mercury acetate to build non-explosive salts.
Concerning the mercury salts, Beilstein says propyne easily reacts when shaken with aqueous solutions of HgCl2, HgSO4, or mercury acetate, with more
difficulty HgBr2, and not with HgI2. What results (from HgCl2, etc) Beil says are precipitates from the propyne, non-explosive mercury salts, which
easily solubilize in HCl, forming acetone. If propyne is led into a solution of HgI2 in KI, which has KOH, added, a crystalline precipitate of
(C3H3)2Hg results (Ber. 17, 13).
(C3H3)2Hg also results from leading propyne through water-containing mercury oxide. This compound is fine glancing needles (from hot alcohol). Unsol.
in water, almost insol. in cold alcohol. Sol. in HCl, forming propyne. It does not explode when heated. With alcoholic mercuric chloride it gives a
crystalline precipitate, which gives off acetone with acids.
2 C3H4.3HgO.3HgCl2:
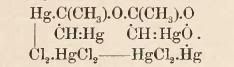
is a fine crystalline precipitate, obtained from leading propyne into a cold mercuric chloride solution. If mercuric chloride solution is hot
(90-95°C) acetone forms immediatley, and this same amount of salt can continously convert propyne into acetone. The complex is insoluble in water,
cold alcohol. Sol. in HCl, forming HgCl2 and acetone. It decomposes quietly when heated. Decomposed by bromine without forming acetylene bromide. 3
C3H4.5 HgO.HgSO4 + 7 H2O - Precipitate. Barely soluble in dilute H2SO4, easily in hydrochloric acid. 2 C3H4.3 HgO.Hg(C2H3O2)2. Amorphous precipitate,
easily decomposed by hydrochloric or acetic acid.
The copper salt, which might have the formula of (C3H3)2.Cu, is said to glow and smolder out when heated. It forms propyne with HCl. It is soluble in
a mixture of NH3 and NH4Cl, so that's why HCl solubilized Cu2Cl2 which is saturated with NH3 that then absorbs propyne, does not form a precipitate.
[Edited on 14-5-2008 by Schockwave]
|
|
|
Axt
National Hazard
   
Posts: 798
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Very interesting Schockwave, with both mercury and copper methylacetylides being non-explosive. Quite a contrast to the acetylides.
So it was likely the methylacetylide that I obtained from a cuprous solution, though that would make it CuCCCH3. The image I attached above has gone
dead so I'll re-attach it. Indeed it glowed and smouldered as in the description you gave. Does it mention a colour?

|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I would like to comment that while MAPP gas is more expensive than propane, it is less than $10 a bottle in the US. Even the "self striking"
attachment is only $20 and it comes with a bottle of MAPP. I mean, I bought a kit that came with MAPP gas and an oxygen cylinder plus a plastic hose
type adapter with a bunsen burner type tip. The O2 and gas flows could be adjusted, as well, to give varying flame types. It was only $40 and it is
very easy to attach a rubber hose to the tip of the torch for the purpose of bubbling through a liquid.
Also, MAPP gas does contain LPG (propane and butane mostly) which usually has a warning chemical (usually a thiol like ethanethiol).
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by Axt Does it mention a colour? |
No color is mentioned.
There's also more on silver methylacetylide in Gmelin Ag [B 5], p. 14: they also say deflagration at 150 deg., but also mention that it can be dried
at 60 to 70 deg. They say the white compound turns yellow-red in light and later grey. They also mention its preparation from AgNO3 in aq. NH3
(probably precipitates much faster). They also say it is not "contact-sensitive". Mineral acids, HS-, and heating with acetic acid releases propyne.
But with Br2, PCl5, and SbCl5 it reacts with ignition. With solutions of I2, AgI and CH3C:.CI (methyliodoacetylene) result, solutions of Br2 react
analogously [C.Liebermann - Liebigs Ann. Chem. 135 [1865] 266/90, 268/71].
And other silver acetylides (p.12-17), these following are just the ones specifically mentioned to be energetic:
AgC:.CC:.CCH3 ( = AgC5H3) is made by reaction of aqueous ammonicial solution of AgNO3 with CH3C:.CC:.CH, where it precipitates. It is yellow-brown,
and extremely shock- and friction-sensitive compound. With conc. HNO3 an explosive decomposition results (H.H. Schlubach, V. Wolf - Liebigs Ann. Chem.
568 [1950] 141/59, 155). The same reference also mentions AgC:.CC:.CCH3.AgNO3 (= AgC5H3.AgNO3) which results from an alcoholic solution of AgNO3 and
CH3C:.CC:.CH. Though nothing was mentioned in Gmelin about its properties.
AgC:.CCF3 (= AgC3F3) is obtained as a white precipitate by shaking a solution of aqueous ammonicial AgNO3 solution with CF3C:.CH. In light it turns
bright brown, with mild warming it eventually decomposes to Ag. But by quick heating it deflagrates violently. It's soluble in organic solvents (R.N.
Haszeldine - J. Chem. Soc. 1951 588/91; Nature 165 [1950] 152/3).
2 AgC:.CCR'R"OH.AgCH3COO (where R' = C2H5, R" = CH3; R' = R" = C2H5; R' = i-C3H7, R" = H), with the sum formulas being AgC:.CCR'R"OH:AgC6H9O, AgC7H11O
or AgC6H9O. The compounds result from alkinols CH:.CCR'R"OH and double the mole amount of AgCH3COO in aqueous solution, where they precipitate as
colorless amorphous solids. They can be dried next to H2SO4, they are explosive and solubilize in dilute HNO3 (Ber., 64 [1931] 2371/5).
Alkoxyalkinyl silver compounds: an Ag-deriative of CH:.COC2H5 precipitates from its interaction with a solution of ammonicial AgNO3 in C2H5OH as a
colorless crystalline solid. It discolors in light forming Ag, but it detonates violently from mild heating. Insoluble in H2O and organic solvents.
The analogous Ag-deriative of CH:.COC3H7 is darker than the ethoxy compound (Zh. Obshch. Khim. 15 [1945] 394/400, 397/8; C.A. 1946, 4657).
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
A dirty grey precipitate results from a solution containing mercuric chlorate and saltpeter and MAPP. I'm not sure about the formula or properties,
though it could be a compound analogous to chloratotrimercuraldehyde. In small amounts it deflagrates vigorously from a flame, confined in Al-foil it
will pop and puff very mildly, even if mixed with KClO3. A real hard hammer blow will cause it to snap. Although this doesn't show that interesting
energetic properties an analogue of far more dangerous chloratodimercuraldehyde might.
[Edited on 26-5-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I've found corroboration for the color of the copper salt. The Journal für praktische Chemie (1866), Vol. 2, p. 177 says that leading propyne into
ammonicial CuCl yields the "well-known" yellow precipitate of copper methylacetylide. It also decomposes from dilute HCl to give off the alkyne. Then
the Handbuch der Chemie (1867), p. 529 says it is siskin yellow. Leading propyne into 2KCl.CuCl (or the iodide analogue) it is richly absorbed and
colors the solution yellow and precipitates bright yellow crystals which are complexates of the copper (I) halide with the methylacetylide.
The latter ref also mentions gold methylacetylide. Made by leading propyne into an ammonicial solution of "unterschwefligsaures Goldoxydul-Natron",
which is just sodium aurothiosulfate Na3Au(S2O3)2 made from mixing aq AuCl with aq Na2S2O3, or made from AuOH mixed with Na2S2O3. For AuOH:
precipitate cold from SO2-decolorized gold salt solution using aq KOH, it's darkviolet and freshly precipitated it is soluble in cold water with blue
color.
Silverpropyne also complexes with sulfate or chloride. But for one that can't be found in the literature that is the silver chlorate methylacetylide
complex, the likley structure is as shown below and which is similar to the chloride analogue mentioned in Annalen 157, 242, possibly as a hydrate. A
perchlorate also likley exists.
It is made by leading propyne into aq. solution of AgNO3 which had KClO3 dissolved into it. It is a whitish solid similar to the AgC3H3, but less
light-sensitive. The dangerous thing is the salt tends to clump even after washing with anhydrous ether. It is shock sensitive (but less than Ag2C2 or
AgN3), over >0.03 Nm. In small amounts exposed to open flame reacts similar to AgC3H3 giving large amount of soot, but if it is under light
moderate confinement it will detonate with quite some brisance and a loud report, unlike the AgC3H3 which puffs lightly with a pop and low brisance.
The reason for the violent reaction may be due to the gases formed on its decomposition including ClO2, in addition to the triple bond energy source.
Added to conc. HCl, it is destroyed giving a Cl2-like odor (probably various gases).
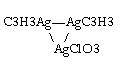
|
|
|
Axt
National Hazard
   
Posts: 798
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
You digging up and posting these references is much appreciated Formatik. I assume the chlorate complex you mentioned is one of your own
experimentation? Sounds like an interesting one to try as an initiating explosive.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Right, and I suspect it has initiator properties also, but it might be inferior to silver acetylide and azide. The regular Cu and Ag salts can also be
oxidized to yield dialkynes.
|
|
|
carbonfeind
Harmless

Posts: 8
Registered: 5-11-2009
Member Is Offline
Mood: No Mood
|
|
I realize that I have yet again revived a year old thread, but I thought it would be better to post this here instead of making a new thread. I hope
that is OK.
I have done a bit of work with the chlorate complex of silver methylacetylide.
It was synthesized as follows:
In a 100ml beaker 2.5 grams of AgNO3 and 1.5 grams KClO3 were dissolved in 39ml of room temperature distilled water.
This solution was then poured into a 100ml graduated cylinder.
MAPP gas from a "bernzomatic" cylinder was led into the solution via a glass tube for about 5 minutes.
The precipitate was allowed to settle at the bottom and as much water was decanted as possible.
It was then filtered and rinsed several times with cool acetone and allowed to dry in the dark.
The silver methyl acetylide-chlorate has an extreme affinity for clumping so care should be taken as to not allow it to dry in large chucks.
The silver methylacetylide-chlorate complex deflagrates in a quick crackling/hissing flash with evolution of black smoke. It appears to deflagrate
slower than the straight methyl acetylide.
If there are lumps present, or the otherwise loose powder is even slightly compacted, it will not deflagrate, but detonate. this gave me
quite a fright once when I first made this compound as I was expecting a "thump" but instead got a sharp "bang". So much care must be taken when
attempting to observe the deflagration.
As you would imagine, little confinement is needed for DDT.
This compound appears to have initiating capability on par with silver acetylide, but no really proper test were done. However, when equal amounts of
equal densities are detonated on a thin aluminum sheet, the dent created by the silver methyl acetylide chlorate is much more pronounced than silver
acetylide.
Friction and impact sensitivity appear to be relatively equal, but once again, no proper testing was done.
I also tried to form the perchlorate complex, but I appeared to be unsuccessful. Both ammonium and potassium perchlorate were tried, maybe a dilute
solution of HClO4 would fare better?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
It's possible I could have underestimated the propyne complex, since it was not as flame and impact sensitive as acetylide-nitrate and azide. The
chlorate gives it obvious extra energy and probably sensitivity. Silver chlorate itself has some energetic properties, i.e. Sodeau found silver
chlorate explodes easily by a hammer blow. Heating AgClO3 to about 350 deg. also caused explosion. With combustibles, AgClO3 also detonates more
easily than KClO3 through shock.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Carbonfiend: Email me at d.martyn@live.ie
Anyways... As for the silver one. Here is an idea... Use Carbonfiends synth for the chlorate double salt, with an excess of silver nitrate. Then add,
with stirring, a mixed basic solution of alkaline sodium picrate and sodium azide. It should form a nice complex...
OT but making a serious excess of AgNO3, adding C2H2 with rapid stirring and heat while also dropping in NaN3 makes what appears to be at least a very
good coprecipitate of Silver Azide-Acetylide. I plan to try this with chlorate soon-ish - I am in the middle of exams - and examine the PPT under
microscopy.
IIRC for good clathrate/co-ppt, we need to keep the cauldron hot, the addition slow, and the stirring fast 
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
One of the production elements in azide materials is to keep precipitate cold (5C) to prevent production of needle crystals. ANY time azide is
utilized, microscopic examination is prescribed to determine if needles are present. If so; the batch should be destroyed. This is from a German &
British patent & SOP from plant production techniques. The microscope should show round-ish particulate of inconsistent shape (even in clathrates)
and any needles are deemed too dangerous to maintain in yield.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
That is true, however I find that it forms fine material - I have not 'scoped it yet, but when I get half a chance I shall. It may well form
micro-needles, but it felt like fine salt to handle. Not quite granular, not powder, just in between. A lot like the Azo Clathrate (4/12) is to
handle. Also, I was thinking of it as 'clathrate' and the rule there is heep it hot and stirred while adding slowly!
I am thinking that Rosco could illuminate us here, but I had an idea (OT again but kinda relavent).
Regarding his Basic Lead Picrate-Chlorate-Nitrate mixture he referred to as a good 'Primary Flash Ignitor' and it is a clathrate material. Would
replacing the Chlorate with Perchlorate, having a huge excess of Lead Nitrate, and then dropping in slowly Sodium Azide under heating and stirring be
the way to form the mysterious 4/16 or 4/17 perchlorate based azo chlathrate?
I wish to test that material, as I have an idea for it to be mixed with DPNA or DPB in a matrix of NC to form small 'cylinders' of 'cast primary'
which would have a bridgewire assembly inserted into them, and then be used as part of the CLD project. DPNA or DPB are both excessively hot - 2.5mg
to initiate PETN! Microtek or PDB can confirm this.
The idea of using the hottest clathrate there is is to help with unequivocal initiatiory performance - i.e. to shorten the DDT in order to enhance
efficiency.
I was thinking, for the testing of interesting primaries, could we devise a 'critical diametre' method of testing? Or some such method...
I already have a test-method devised (a series of 'benchmarks' however to test a range of primaries, and repeat each test 3-5 times as required by
proper scientific method, would be an undertaking similar to that of a Masters thesis or PhD. Perhaps I will do it for my Masters or PhD 
There are literally tens of thousands of potential tests - and they would all help fill in the fragmented picture of how these interesting materials
function!
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
pdb
Hazard to Self
 
Posts: 83
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
Talking about needles and DPNA, I obtained very regular micro-crystals by adding NaNO2 as powder iso aqueous solution (which leads to bigger crystals,
irregular both in size and shape). From a macro viewpoint, the product prepared this way is easier to handle, although still not really free flowing.
(I'm puzzled by your "DPB".. what does it stand for ?)
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
I will find the other name for DPB, but basically DPNA except replace the 3-nitroaniline with 2,4-nitroaniline. IIRC you made something of the sort.
INCREDIBLY potent primary, and I thank you for introducing me to DPNA and such.
More on the topic, today I found interesting article about making double salt of Ag2C2:AgClO4. The article is moreso about the crystal structures but
I think it is giving me ideas that Ag2C2 could be used as a basis for a clathrate type mixture.
I will try attach article now...
Attachment: acetylide-perchlorate double salt.pdf (94kB)
This file has been downloaded 1241 times
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
| Pages:
1
2 |
|