| Pages:
1
2 |
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I fancy the idea of CO2 being a replacement for some reactions. I've often wondered though, in general can it prevent oxidation by the mechanism of O2
+ Heat for some of the more prone compounds(provided the purge/blanket/flow is sufficient)? I've assumed yes, however, I've seen very little mention
of it in journals, and forums.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by MagicJigPipe  | | And I don't consider argon very expensive unless you're comparing it to CO2 or maybe N2. You can buy 20 ft^3 (I assume that's at STP) for about $10.
I am constantly surprised at how much that is.[Edited on 12-26-2011 by MagicJigPipe] |
Argon is relatively inexpensive because it's a by-product of the distillation if air. In a sense, it should be cheaper than CO2:
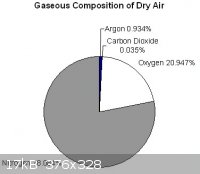
Of course, CO2 is cheaper because it is easily sequestered from localised high volume sources, like chemical plants for example.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
paulr1234
Hazard to Self
 
Posts: 51
Registered: 30-8-2010
Member Is Offline
Mood: No Mood
|
|
Now relocated to the USA, I'm not sure what I am most jealous about, Peach's bottle of Abbot or his 60lt SIP bottle of Argon, nether of which is easy
to find State-side.
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
I was not aware of the existence of those disposable argon bottles... strangely, they only seem to be available in Australia/NZ... I've been thinking
for a long time about outfitting myself with an argon bottle and regulator, but I was put off by the outrageous prices!
Maybe this could be an interesting alternative. A small disposable cylinder could probably last me one year & +...
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
paulr1234
Hazard to Self
 
Posts: 51
Registered: 30-8-2010
Member Is Offline
Mood: No Mood
|
|
Sold by Halfords in the UK for about 13 quid by the looks of things.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
If you google, 'disposable argon cylinder', 'disposable mig cylinder' or something along those lines, it'll churn out a load of different places.
Toolstation is the only name required.
If I was in the US, I'd definitely grab one of these from Harbor Freight.
20 cubic feet, 5" by 15" (smaller than your monitor) makes it nice and compact but it'll have enough in it to last a long time, all DOT coded to avoid
the risk of DIY pressure vessels, $84.99. Perfect. Stick a regular on there and you're flying.
<img src="http://img52.imageshack.us/img52/8452/image13676.jpg" width="200" />
Sign up at CNCZone.com and Weldzone.com. You can also try Weldingweb.com. There'll be loads and loads of guys there who can help you out with
cylinders and filling (particularly if you're in the US). A lot of them have the latest gear from ESAB and Miller, and access to plasma / laser /
waterjet tables, serious CNC mills and lathes. I think CNCZone has a job section, on which you can list up your required part and find someone to sort
it for you.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size(s)]
[Edited on 17.12.13 by bfesser]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
While we are on the topic of inert gases, I will also mention sulfur hexafluoride . It is surprisingly inert, not even reacting with molten elemental sodium (it does not react until the boiling point is
reached). It is also safe to breathe, provided of course that it is premixed with oxygen, otherwise it can easily cause suffocation.
Some of the few things that SF6 does react with at lower temperatures are a solution of sodium in anhydrous liquid ammonia, or sodium in the presence
of diphenyl ethylene glycol dimethyl ether. Hydrogen sulfide can suppossedly also reduce it. Hydrogen iodide reduces SF6 at room temperature.
"Reaction between Sulfur Hexafluoride and Hydrogen Iodide", D. K. Padma, A. R. Vasudeva Murthy
Obviously elemental sodium is a stronger reducing agent than HI, but the differences in reaction in this unusual situation are due to steric reasons.
A cylinder of SF6 can be purchased for around 250 euros.
Carbon tetrafluoride is also an inert gas, although it reacts spontaneously with elemental lithium or sodium.

[Edited on 30-12-2011 by AndersHoveland]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Of course, CO2 is cheaper because it is easily sequestered from localised high volume sources, like chemical plants for example.
|
It also condenses at a much higher temperature (-78 °C) than argon (−185.85 °C)
In terms of the latent heats of vapourisation, CO2 does involve a larger change (15.326 kJ/mol at –57.5°C) than argon (6.43 kJ·mol−1). But
creating and maintaining the temperature differential for argon consumes a lot of energy.
Contrary to the films, it takes about 18h to freeze a human body to liquid nitrogen temperatures because the latent heat for vaporising nitrogen is
about 5 kJ / mol. Water is 40.7. Hence the serious burns that result from steam and boiling water contacting skin, versus spilling liquid nitrogen.
The air temperature at lake Vostok has reached -89.2C (making it the coldest place on earth).
Do you get to keep the cylinder, or is that per fill? I seem to remember SF6 being quite expensive. Even if you get to keep the cylinder, a fill of
argon is about £50-75.
I just remembered, there are two other places you can try for inert gas and refills.
Paint ball people use CO2. They'll usually have one or more shoulder / head height cylinders and the people there are constantly refilling the
cylinders. Some of them also use high pressure air or nitrogen. A 20oz paintball gun cylinder is about £30.

Divers and dive shops also tend to have cylinders near by. Numerous different gas blends exist for different dive profiles, as regular air induces
nitrogen narcosis below about 30m. Deeper down, oxygen becomes toxic. The stores will produce blends of gases for technical diving.
Nitrox - Air with extra oxygen and bit of argon, to extend the length of shallow dives
Argox - Argon and oxygen
Heliox - Helium and oxygen
Hydrox - Hydrogen and oxygen
Hydreliox - Hydrogen, helium and oxygen
Trimix - Helium, oxygen and nitrogen (most technical divers use this for 100m dives, which is about the limit)
So the shops will have those cylinders sat around for the blending. As well as high pressure compressors for charging them.
Divers sometimes use argon to pressurise their dry suits, as the lower thermal conductivity of it versus breathable air helps keep the cold away. Or
argox, or oxygen, to avoid having nitrogen under pressure in contact with their skin at depth. Armed with one of those harbor freight 20 cuft
cylinders, call and ask about gas blending, if they do it or who else to call, then say you've got a small suit filling / bail out sized bottle and
ask if they can charge it up with pure nitrogen or argon.
The affectionately named 'yellow box of death' rebreather. Five thousand buttons to press and hoses to tangle in whilst you suffocate in the cold,
cold dark - trapped.

A typical technical diver, going for a relaxing swim.

[Edited on 30-12-2011 by peach]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Even nitrogen gas is not completely chemically inert. Although it will not react with elemental sodium or potassium at any
temperature, it will gradually react with metallic calcium at room temperature.
| Quote: |
At normal temperature, Ca rapidly acquires a layer of nitride and oxide, but forming chiefly nitride. At higher temperature both CaO and Ca3N2 are
formed.
|
"Concise Encyclopedia Chemistry", deGruyter
| Quote: |
Someone left some calcium in my glovebox in an open container. The glovebox was obviously 100% nitrogen environment. The box also happened to be at
~30 C at the time. By the next day it was all calcium nitride.
|
"enahs" from "Chemical Forums"
Interestingly, metallic calcium does not actually react with anhydrous ammonia at room temperature, but rather dissolves in the cold liquid. When
heated (or with the use of an iron nitrate catalyst) it forms calcium nitride and calcium hydride, which are very different products from the
reaction of sodium and NH3.
6 Ca + 2 NH3 --> Ca3N2 + 3 CaH2
2 Na + 2 NH3 --> 2 NaNH2 + H2
Nitrogen is actually soluble (only slightly) in molten sodium. "N-Na (Nitrogen-Sodium) System", James Sangster, Journal of Phase
Equilibria and Diffusion
Volume 25, Number 6, 560-563,
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Interesting note there Anders.
It also underlines what I've been going on about, that if nitrogen can cause issues, you don't want to be messing around with things that are a lot
more reactive in search of inert conditions. Argon is the way to go.
| Quote: | Argon’s complete octet of electrons indicates full s and p subshells. This full outer energy level makes argon very stable and extremely resistant
to bonding with other elements. Before 1962, argon and the other noble gases were considered to be chemically inert and unable to form compounds;
however, compounds of the heavier noble gases have since been synthesized. In August 2000, the first argon compounds were formed by researchers at the
University of Helsinki. By shining ultraviolet light onto frozen argon containing a small amount of hydrogen fluoride, argon fluorohydride (HArF) was
formed.[2][16] It is stable up to 40 kelvin (−233 °C). The ArCF2+
2 metastable dication was also observed.[17] |
I'd have to put effort into achieving that. As opposed to hydrogen and propane, which will happily explode with the slightest mistake.
[Edited on 31-12-2011 by peach]
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
i use a Air Liquide cylinder of compressed balloon grade helium for all of my reactions that have to be run under inert gas as well as for my gas
chromatographs. I successfully prepared cobaltocene using this gas, this compound is extremely oxygen sensitive and potentially pyrophoric.
Amateur NMR spectroscopist
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
No one seemes to mention the degredation of the planets atmosphere by recklessly using copious amounts of co2 and methane, ethane, propane, butane. It
may be inert to you but what about the trees and the orangutangs. I think gm is wrong but sometimes i can't afford the organic alternative. How much
is organic argon a cylinder?
Well if no one else cares i'm not going to either, ( cue music, 'arsehole' by denis leary)
can't wait for my burger.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
And how else are we supposed to cool down our swimming pools when they've burnt too much fuel and become too warm?
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/uhXA9ON6igk#t=01m15s" frameborder="0" allowfullscreen></iframe>
[Edited on 3-1-2012 by peach]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Solubility of nitrogen and the noble gases in molten sodium and potassium:
"Solubility and Diffusivity of Inert Gases in Liquid Sodium, Potassium, and NaK", E.L. Reed, J,J. Droher
http://www.osti.gov/bridge/servlets/purl/4181890-sqUlyW/4181...
Realise that this does not mean nitrogen is unreactive at higher temperatures, just that there are unique chemical reasons that nitrogen does
not readily combine with sodium or the alkali metals below it. Magnesium, a less reactive element than sodium, can burn in pure nitrogen. And
nitrogen would also not be soluble in an amalgam (liquid mercury alloy) of aluminum or titanium, as it would instead react.
| Quote: |
"Dinitrogen has been known for many years to react with metals, such as lithium amongst the alkali metals and calcium, under very mild conditions.
Such reactions are recognized to be initially surface tarnishing reactions, and the ultimate bulk product is the metal nitride, such as Li3N."
Nitrogen Fixation at the Millennium, edited by G. J. Leigh
|
[Edited on 28-4-2012 by AndersHoveland]
|
|
|
Digital Hepatitis
Harmless

Posts: 12
Registered: 20-5-2012
Member Is Offline
Mood: No Mood
|
|
You can easily get pure argon gas as "wine preserver". This is a much more cost-efficient way of acquiring this than going down the welding canister
route, if you are so inclined. If you only need a small amount of inert gas you can easily pick up a canister or two of wine preserver (just ensure
that your brand is pure argon gas, as some are a mixture of gases.)
|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
Refreshing an old thread but better than making a new one eh?
I would love to have an inert atmo available to work under in my lab, however, I have not been able to find very cost effective routes to this.. Yes
you can get Ar from Harbor Freight for $84.99 (as of today), but then the regulator is going to cost how much... another $100-$250?
There are welding supplies in my area but they only offer huge tanks (the 5'+ size) and those are not affordable..
I seen someone mentioning R-134A in the thread, and while Not cheap in my area ($11 for a small can minimum), is easily available and regulators are
not hard to come by (I have two).
So, for someone that performs maybe 3 (or wants to) inert atmo reactions every year (at this point), what would you guys recommend. I can pick up a
big tank of He, but being lighter than air you would start bottom up, but am not sure about reactivity without more research which I can do of course,
but am now looking for an overall inert gas.
So TLDR;, is He viable for grignards? CO2 is definitely out for carboxylic acid production. Has anyone found a viable N2 route that doesn't cost $300
US to purchase, same for Ar...
Thanks for any input 
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
If you're doing your Grignard in ether reflux, the ether vapour is all the inert gas you need.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
If anyone does manage to find a cylinder of argon or helium, I think I have some regulators for them. Since I don't have any cylinders at home, I
haven't tested them out yet, but I plan to sort through them one of these days. While they are expensive to buy, there seems to be little demand for
used ones, so my price would be low. I always liked argon, but N2 works fine for most things. It is cheap to get cylinders refilled with, once you
have a cylinder. (At least it used to be.)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's some more info:
http://www.sciencemadness.org/talk/viewthread.php?tid=10366#...
Refills are cheap in my locale.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
Yes, just buy some dry ice and sublimate it. It costs $1 per pound, and is easy to get.
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Please refer to my signature...
Also, CO2 is useless for many reactions, like Grignards. It is also relatively soluble in water and it is acidic. So yeah, dry ice might be viable for
those who can get it when you need something for a reaction where it will work. But investing in a CO2 bottle would just be stupid if nitrogen or
argon is available at a similar price.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
TheChemiKid
Hazard to Others
  
Posts: 493
Registered: 5-8-2013
Location: ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
Member Is Offline
Mood: No Mood
|
|
That is true. I was just adding carbon dioxide as a choice because buying dry ice is cheap, and is better than just plain air for most reactions.
When the police come
\( * O * )/ ̿̿ ̿̿ ̿'̿'̵͇̿̿з=༼ ▀̿̿Ĺ̯̿̿▀̿ ̿ ༽
|
|
|
| Pages:
1
2 |