| Pages:
1
2 |
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I was wondering how good is the magnesium acetate for pyrolysis.
I've made an attempt to heat 0.5 mole (40 g) of magnesium acetate at 350-400°C using a slight flow of CO2 (approx 0.3 L/hour) to sweep the gases from
reactor. This led to 35 ml of distillate (magnesium acetate was not completely anhydrous) and a lot of fumes easily passing condenser and making
clouds in the receiving flask. Distillate had no layers (even after I've added NaCl to salt out acetone), but the liquid had yellow color at top,
while bottom was completely clear.
After I've added NaCl, small amount of NaHCO3, 15 ml of ethyl acetate, I mixed this stuff a bit and then it separated into 25 ml of top yellow layer
(+10ml of something) and clear bottom layer. The top yellow layer has horrible smell.
I remember I tried to make acetone by oxidation using NaOCl + acetic acid, and I've got pretty much the same stincky liquid plus a lot of clouds of
non-condensable compound when the source flask was heated strong enough (there was some non-volatile liquid left, so I was trying to distill it too).
What was the volatile gas? Could it be a ketene? I have no idea.
If I assume that the acetone moved to the ethyl acetate layer and all the 10 ml volume is acetone, then I've got 8 g/130 mmol of acetone, which is 25%
yield. Probably I should have used more strong current of CO2, something like 2L/h. Or maybe make sure the magnesium carbonate has no traces of acetic
acid.
I'm gonna try to perform fractional distillation of the ethyl acetate part.
[Edited on 30-7-2015 by byko3y]
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Interesting experiment.
What sort of "horrid smell" did you feel?
Was it kind of suffocating e.g. like formaldehyde? Was it simply nauseating like a decomposing dead rat in a storage box?
Of course the answer will not shed more light on what the reaction produced, I'm simply curious. :-)
The use of Mg is interesting, though in the literature they prefer Ca or Ba if I remember well.
How do you plan to investigate the product further?
|
|
|
kecskesajt
Hazard to Others
  
Posts: 299
Registered: 7-12-2014
Location: Hungary
Member Is Offline
Mood: No Mood
|
|
Maybe it wasn't so inert gas or had other metals in there and the acetone pyrolised into ketene.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Finally I've finished the separation of the product. I needed to calibrate my distillation column, it appeared to provide 3-5 theroretical plates (40
cm vigrex column with), so I needed at least 2 distillations to obtain a relatively pure acetone.
So the isolated yield is 1.5 ml (20 mmol) out of possible 18 g (300 mmol). Also, I've got 2.5 ml of some high boling liquid, wich contains acetic
acid, I'd say it has 1.5 ml/13mmol of mesityl oxide. I'm pretty sure the actual yield of acetone was 3-4 ml, but I've lost some amount during
purification. Anyway, it's 10-15% yield of acetone plus 10% yield of polymertization products (100% yield is a quantitive conversion to either acetone
or polymers), and 80% decomposition products.
Pumukli, the smell is very similar to the acetone, some heavy slightly irritating flower odor, it felt like the whole house was
filled with acetone, although I'm sure my device had no leaks - the clouds were coming from the outlet, and not anywhere else. And yes, I felt some
dizziness breathing those gases. I would not say it's very similar to an odor of decomposing body.
Calcium acetate requires at least 400°C for decomposition to take place, and 400°C is the upper limit for my glass devices. I could rich 500-550°C
by heating from all sides, but for now I have only a regular heating plate, and I've detected it to be as hot as 450 °C while the temperature inside
the flask was barely reaching 400°C. I think some amount of polymers were left in the flask (it had the characteristic odor of polymeric products).
I'm gonna try to use a stronger flow of CO2 through the mixture, also I'm gonna need a higer temperature because the gas will cool the reactor. I did
not dry the magnesium acetate well, this might be another reason for the failure.
AFAIK, MgO is a relatively strong base, but Mg(OH)2 is insoluble in water, that's why its solution in water cannot be really basic, but the freshly
prepared (activated) magnesiu is caustic, that's why it seems readily cause polymerization.
I was not able to find data for decomposition of barium acetate and nobody seems to ever try it. I'm not really willing to perform the experiment
because barium has a notable toxicity. Thorpe mentiones that calcium acetate needs higher temperature for decomposition, patent US 648,389 tells about
300°C for barium salt and 400°C for calcium salt. Here's a picture for calcium acetate:
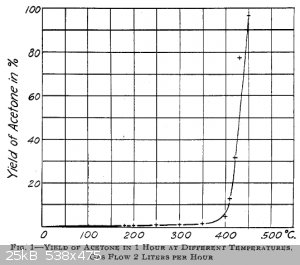
UPD: some more data on pyrolysis temperatures:
http://pubs.rsc.org/en/content/articlelanding/1952/jr/jr9520...
DOI: 10.1039/JR9520001383
"Experiments with non-isotopic materials showed that the decomposition of barium acetate proceeded rapidly only at temperatures above 440°C. In the
pyrolysis of calcium acetate Ardagh, Barbour, McClellan, and McBride (Id. Eng. Chem, 1924, 16, 1133) reported that this reaction only became rapid at
temperatures around 430°C although small traces of acetone were formed at temperatures as low as 160°C".
[Edited on 5-8-2015 by byko3y]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
How do you currently condense acetone in the flow of carbon dioxide? If you increase the flow, you will have to use a trap cooled with a dry ice in
acetone bath. I mean, obviously you cannot use cold water as the partial pressure of acetone is about 90 mbar even at 0 °C. It therefore takes a very
low flow of carbon dioxide to obtain a partial recovery of acetone with ice cold water cooling. With the flow increased you will not be able to
condense it with cold water.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
In fact I was thinking about that when deciding what flow I should use. Later I forgot about this problem, and authors of the articles I have read
just used hypohalite/bisulfite traps.
In fact I was using 25°C water for cooling, and acetone has insane 200 mmHg vapor pressure at this temperature. During the experiment I was trying to
cool the water little bit but I got only 10-15°C and this led to 150 mmHg vapor pressure. While amount of CO2 used was 0.3L/h, the decomposition
products had much higher volume, and if we suppose their amount to be 20% of the molar amount of acetate, than their volume was ~2L.
2.3 L can carry up to 26 mmol of acetone vapors, which is the half of the yield I've got, and this used to be a common reason for lower than
quantitive yield of acetone in former times.
My data tells me that acetone vapor pressure at 0°C is 70 mmHg, which is acceptable for 2-3 L/h gas flow (10 mmol/0.6g loses per hour). Most basic
water pumps are made from plastic, and for polypropylene the lowest temperature is -15..-5°C. A slightly salted water with ice will provide -5°C (50
mmHg acetone), and I would not bother catching the residual vapors.
[Edited on 5-8-2015 by byko3y]
|
|
|
| Pages:
1
2 |
|