| Pages:
1
2 |
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
OK
If i want some pure ethanol there is a product avaliable that contains this according to the safety datasheet.
Ethanol >75 - 100%
propan-2-ol 10 - <20% (isopropanol)
butanon; 0,1 - <1% (ethylmethylketon)
acetone 0,1 - <1%
Is there any easy way to purify this so i get pure ethanol for my chemistry experiments?
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
You can make very pure EtOH by fermentation and fractional distilation. Look at Fery's thread about it, I run his EtOH through GC, it contain just few
tens of ppm MeOH and higher alcohols. I can post reports if desired.
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Thats nice tnx, i look into it.
I just looked at various etanol products but all contain what i specified in above post.
Exept hand disinfection alcohol, it contains just Etanol 629 g/kg and Isopropanol 63 g/kg.
But they are maybe tricky to separate.
I probably try the fermentation route to ethanol.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
EtOH and i-PrOH can be separated by fractional distilation. But I don't have any experience with it.
Fery's thread is here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
And here are reports from GC. He sent to me 3 fractions, all are pretty pure, the biggest impurity is MeOH, but its content didn't exceed 0,1% (MeOH
is at retention time 1,5; everything else after EtOH are probably higher alcohols, in one fraction there was also some ethyl acetate).
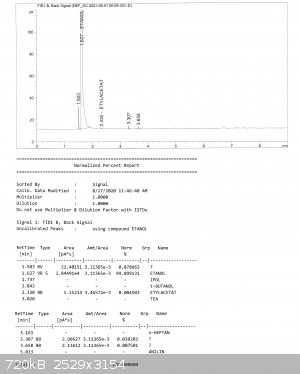
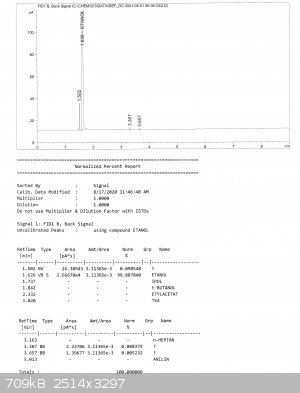
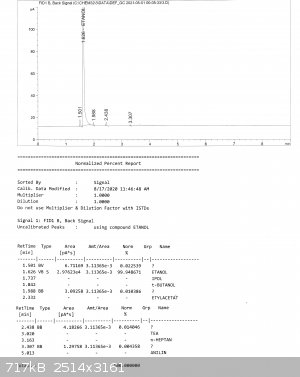
[Edited on 17-1-2023 by Bedlasky]
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Wow, 99.9% and 99.95% ethanol.
Getting that % from fermentation and distillation is impressive and this quality of ethanol should do for almost all things.
I have made a comment in Fery´s original thread about adding a step with active carbon treatment.
|
|
|
SuperOxide
Hazard to Others
  
Posts: 487
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Oh boy. Well. After starting with close to 1.5L of denatured ethanol, and processing it four or five times with very generous amounts of KOH and NaOH,
I can say the 700mL I got out on the other end substantially has so little amount of denaturant in it, that I can't smell it one single bit :-)
I'm also coming to the conclusion that it's so much easier/cheaper/faster/cleaner to just purchase everclear....
YOU WIN, UNCLE SAM! lol.
|
|
|
Nitrous2000
Hazard to Self
 
Posts: 59
Registered: 1-11-2013
Member Is Offline
Mood: No Mood
|
|
denatured ethanol
hi guys,
I see from this thread that removing Bitrex from alcohol may be unfeasible but another source of alcohol (50% or so) are alcohol solutions tainted
with propylene glycol.
This is commonly sold as antifreeze for potable water systems.
Nitrous
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mateo_swe  | Wow, 99.9% and 99.95% ethanol.
Getting that % from fermentation and distillation is impressive and this quality of ethanol should do for almost all things.
I have made a comment in Fery´s original thread about adding a step with active carbon treatment. |
It also seems impossible w/o 'breaking' the EtOH/water azeotrope?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bedlasky  |
And here are reports from GC. He sent to me 3 fractions, all are pretty pure, the biggest impurity is MeOH, but its content didn't exceed 0,1% (MeOH
is at retention time 1,5; everything else after EtOH are probably higher alcohols, in one fraction there was also some ethyl acetate).
[Edited on 17-1-2023 by Bedlasky] |
Does the GC detector detect water?
(It looks like an FID trace to me)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No detection, no water. Simples!  
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Bedlasky  |
And here are reports from GC. He sent to me 3 fractions, all are pretty pure, the biggest impurity is MeOH, but its content didn't exceed 0,1% (MeOH
is at retention time 1,5; everything else after EtOH are probably higher alcohols, in one fraction there was also some ethyl acetate).
[Edited on 17-1-2023 by Bedlasky] |
Does the GC detector detect water?
(It looks like an FID trace to me) |
No, it doesn't. As you say - it have FID detector, so it can't detect water. We have in job one GC which have different detector and can detect water
(it is used for water determination in aniline and cyclohexylamine). I determined water content on KF titrator. According to KF titration it have 6,5%
water, but Fery's distilate came over exactly at boiling point of azeotrope, so I don't trust this value much.
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Im more intrested in the contaminating stuff than water content.
Its not so hard to remove water up to 95% and if absolute ethanol is needed it can be done as well.
Removing contaminants are harder if they have similar boiling points.
But it looks like Fery´s procedure give very low levels of contaminats so i think thats the way to go for me.
Buying drinkable ethanol is an option but there is probably some contaminants in this too, just not any that is dangerous when consumed.
There are a few places that sell absolute ethanol at higher cost, it might be worth it depending on how one values the time and effort it takes to get
to absolute ethanol with DIY routes.
To get 95% pretty pure ethanol maybe distillation of drinkable ethanol is an option.
|
|
|
| Pages:
1
2 |