| Pages:
1
2
3 |
DennyDevHE77
Hazard to Others
  
Posts: 168
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Where do ETH synthesis recipes come from? I mean, for example, in Urbanski there is a three-parameter diagram of the areas of formation of PETN,
according to which ideal proportions have long been drawn up for which the maximum yield is achieved with a minimum of reagents. And according to ETH,
both on the forum and in various scientific articles, the quantities of reagents are taken as if at random. Hasn't a similar schedule been drawn up
for ETH yet?
|
|
|
Etanol
Hazard to Others
  
Posts: 218
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  | | And according to ETH, both on the forum and in various scientific articles, the quantities of reagents are taken as if at random. Hasn't a similar
schedule been drawn up for ETH yet? |
The chemical properties of ETN are similar to nitroglicerine or hexanitromannitole. It does not have stable sulfoesters, unlike PETN, judging by
scientific articles. Therefore, the acid proportion are calculated with the same force (% H2O).
Quote: Originally posted by DennyDevHE77  | | for example, in Urbanski there is a three-parameter diagram of the areas of formation of PETN, according to which ideal proportions have long been
drawn up for which the maximum yield is achieved with a minimum of reagents. |
The description of the diagram in Urbansky for PETN is incorrectly copied from the source. Do not look at her.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 168
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Etanol  | Quote: Originally posted by DennyDevHE77  | | And according to ETH, both on the forum and in various scientific articles, the quantities of reagents are taken as if at random. Hasn't a similar
schedule been drawn up for ETH yet? |
The chemical properties of ETN are similar to nitroglicerine or hexanitromannitole. It does not have stable sulfoesters, unlike PETN, judging by
scientific articles. Therefore, the acid proportion are calculated with the same force (% H2O).
Quote: Originally posted by DennyDevHE77  | | for example, in Urbanski there is a three-parameter diagram of the areas of formation of PETN, according to which ideal proportions have long been
drawn up for which the maximum yield is achieved with a minimum of reagents. |
The description of the diagram in Urbansky for PETN is incorrectly copied from the source. Do not look at her. |
Thank you Ethyl alcohol, I somehow knew this, but I never thought that the calculation should be different. In general, ETN forms really unstable
sulfonic esters, which break down even with slightly warm water, not to mention boiling in hot isopropanol or acetone.
And what do you mean by Urbansky's scheme? I did not see any description there, only a brief explanation of the regions and that's it. But
nevertheless, according to this scheme, they made a calculation for a sulfur-nitrogen mixture, with high yields. And an attempt to change the
proportions leads either to the waste of extra reagents or to a decrease in the yield (and sometimes oxidation).
[Edited on 14-8-2023 by DennyDevHE77]
|
|
|
Etanol
Hazard to Others
  
Posts: 218
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
And what do you mean by Urbansky's scheme? I did not see any description there, only a brief explanation of the regions and that's it. But
nevertheless, according to this scheme, they made a calculation for a sulfur-nitrogen mixture, with high yields. And an attempt to change the
proportions leads either to the waste of extra reagents or to a decrease in the yield (and sometimes oxidation).
|
The description of the regions in Urbansky has nothing to do with the experiment.
Region 4 is a region of oxidation and zero output.
Region 1 is a region of thermally unstable nitros mixtures, in which there is little sulfoesters, but the heating of the mixture is dangerous.
Region 3 is a region of high stable mixtures in which many sulfoephirs are formed, but they can be heated for converting sulfoesters into PETN with a
high output.
Line CD and to the left - is a region where the reeterification changes the direction. Nitroesters turns into sulfoesters.
The region 2 is unknown.
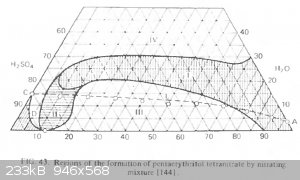
[Edited on 14-8-2023 by Etanol]
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 168
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
It turns out that the very well-known ratios of pentaerythritol (for 70 65 and 58%) of nitric acid were already established taking into account these
typos?
|
|
|
Etanol
Hazard to Others
  
Posts: 218
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  | | It turns out that the very well-known ratios of pentaerythritol (for 70 65 and 58%) of nitric acid were already established taking into account these
typos? |
It seems yes.
I could not find an original article referred by Urbansky. Therefore, I put experimental points on this diagram.
All three well-known recipes (for 70 65 and 58% nitric acid) fall into the region of thermally unstable mixtures. However, at low temperature, they
provide 80-90% outputs of a stabilized PETN and in region 90-95% HNO3 - over 90%.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
I'm giving my alleged 'ammonium nitrate' cold packs that I got a while ago a try to see if they actually are AN instead of CAN. Going to try to
nitrate ETN your way.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
Wow, after almost four months, I finally decided to this, I got some ammonium nitrate synthesized correctly (and very pure) and I am doing using your
proportions. 10g of sulfuric acid and 4g of ammonium nitrate to 1g of erythritol. So far I washing out my yield.
I will edit my post with the yield after it dries.
Edit: Bleh. Only 18.5g of crude ETN (albeit it is extremely white and looks very pure). I think it is because I put it in a solid block of ice when I
first started. from a starting 10g of erythritol. While it is obviously not as high as I wished, it is the best I have gotten so far.
Next time I will not use an ice bath, but I will keep ice handy to control heating if needed.
[Edited on 4-2-2024 by ManyInterests]
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
I did another one that is scaled up. 10 times the sample described here. It was the single most boring nitration I have done. Every other step is
exactly as you described. Let me see what I get.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
In my experience with this preparation, it doesn’t seem to scale well for some reason. I recently switched to the nitric acid method because it’s
so much easier to work with, and gets better yields.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Sir_Gawain  | | In my experience with this preparation, it doesn’t seem to scale well for some reason. I recently switched to the nitric acid method because it’s
so much easier to work with, and gets better yields. |
Oh yes! I just dried the crude ETN and out of 100g of erythritol, 400g of AN, and 1000g of H2SO4 my yield was a paltry 124g
This is really, really bad. I have no idea how I could have gotten that low a yield.
I want to try again. But not with 100g, just 50g of starting erythritol...
[Edited on 8-2-2024 by ManyInterests]
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
I’ve had bad yields with as little as 20g erythritol. This reaction really doesn’t work well above 10g.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
I believe you wholeheartedly now. I did have a problem with scaling up a lot of stuff. Like with potassium chlorate last summer, I made some major
upscaling of production (I got a 4 liter container) and I somehow got 50% yields on the theortical. I guess the higher up you go, the harder it gets
to get good yields.
I think it ceases to be a lab scale issue and more of a chemical engineering issue.
[Edited on 8-2-2024 by ManyInterests]
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
I think this preparation could work if someone did a lot of experimenting to optimize it for a larger scale. It’s been adjusted to produce the best
yield at a 10g erythritol scale.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Sir_Gawain  | | I think this preparation could work if someone did a lot of experimenting to optimize it for a larger scale. It’s been adjusted to produce the best
yield at a 10g erythritol scale. |
I will be doing some experimentation then. Right now I will repeat the same synthesis with the same level of reagents... but I will do something a
little different. Instead of doing it in a beaker, I will do it in a glass loaf pan. It's thick, borosilicate, and will give a wider surface area.
I need to mention that when I moved my thermometer around in the thick sludge it was warmer in some areas than others, this is despite as much mixing
as possible (it was so thick that the stirbar did little to move the whole thing. So I had to use my thermometer adapter to move the sludge around as
much as possible) there were always temperature differences.
But I think the wider surface area might help to alleviate that. On top of that, what I will do is also let it nitrate for a longer time, maybe also
let it get a little warmer once everything has stabilized.
Am I being an amateur as usual or am I onto something?
|
|
|
fx-991ex
Hazard to Others
  
Posts: 109
Registered: 20-5-2023
Member Is Offline
|
|
I think some people doing bigger batches will treat with sulfuric acid first then add nitrate/nitric acid.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
I remember a thread before where someone put their erythritol in sulfuric acid first but got nothing. Wouldn't the acid destroy the erythritol?
Also I need to correct myself. My previous batch was still moist when I first measured it. It was not 18.5g. Now that it is completely dry, it is
actually 15g. Which only adds to my disappointment that I was expecting 22g.
On a plus note, using a nitrate salt (ammonium nitrate in this case) is no different than using regular nitric acid, which makes it a cheaper process
at least.
I will try to do a 50g nitration this way. Same proportions, but I will put the erythritol in the sulfuric acid first and after cooling it I will
slowly add the ammonium nitrate.
Edit: I reviewed the video and I looked at the chart used. He did have a starting 120g and ended up with with a 228g yield. Proportions were the same,
but the starting temperature was at 27C. But no mention of a max temperature
This could be a way to go... simply slowly (and carefully) at the erythritol and let things cool back down. That could work. He did that with impure
ammonium nitrate. I am working with purer stuff.
[Edited on 9-2-2024 by ManyInterests]
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
I tried adding the erythritol to sulfuric acid, then adding nitric acid, and got a horrible yield. Also, using a nitrate salt isn’t the same as
nitric acid. The nitric acid method works far better, and is generally worth your time. You don’t need fuming nitric acid, 70% works great. Using
nitric acid, I get 20g of ETN from 10g erythritol after recrystallization.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
dettoo456
Hazard to Others
  
Posts: 255
Registered: 12-9-2021
Member Is Offline
|
|
Would you choose the azeo HNO3 even over NH4NO3? Industry (or at least BAE systems) seems to prefer the AN/H2SO4 method over mixed acid even. And AN
is generally cheaper than azeo HNO3. Though, I can procure 55gal of 68% HNO3 for $700 from a US supplier, but I cannot find any supplier of NH4NO3 in
bulk or semi bulk at a cheaper price (on a $/NO3 mole basis).
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
Definitely. It’s much easier. The reaction mixture is much less viscous, and magnetic stirring works. I did the math, and it’s about the same
price for me for both methods. I don’t mind distilling nitric acid though, and not everyone has a good distillation apparatus.
[Edited on 2-10-2024 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
In all honesty, azeotropic HNO3 is superior. I did all my previous ETN synthesis using 65 to 80% HNO3. My yields were never super good. I think my
absolute best was getting around 85g from 50g starting erythritol (1.7g per 1g) which was great. I was never able to get 20 from 10g. I simply wanted
to give NH4NO3 a chance at this. HNO3 is good because while the ETN nitration solution will turn milky white and will thicken, it will never become
the sludge that it is in liquid HNO3. Stirring through it in the end was tough because it was like churning butter.
I am thinking of just one more attempt with this method. I got a borosilicate bowl that I intend to use as a nitration vessel. I will make a similarly
large nitration like last time. If I get similarly poor yields. I won't bother with NH4NO3 for ETN anymore.
I am also thinking of simply not doing my intended PETN synthesis with NH4NO3. I will do it once or twice, but if I get yields like I am getting now,
I will bite the bullet and simply make WFNA for it. It would be easier. I can make an abundance of NaNO3 very cheaply to make a lot of nitric acid to
make the PETN I want using 90-99% HNO3.
| Quote: | | I tried adding the erythritol to sulfuric acid, then adding nitric acid, and got a horrible yield. Also, using a nitrate salt isn’t the same as
nitric acid. The nitric acid method works far better, and is generally worth your time. You don’t need fuming nitric acid, 70% works great. Using
nitric acid, I get 20g of ETN from 10g erythritol after recrystallization. |
What are your proportions of 70% HNO3 and sulfuric acid (mine is from 95-98%) to what erythritol? I used 6ml of sulfuric acid, 4ml of nitric acid, to
1g of erythritol previously. Got that from Ballard's videos.
[Edited on 10-2-2024 by ManyInterests]
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 168
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
That's just the same PETN is more profitable to get on azeotrope. The costs of sulfuric acid there are small.
24 ml of sulfuric acid 34 nitric acid 65% and 10g of pentaerythritol give a stable yield of 90-92%.
93-99% nitric acid yields 95-98% of the pure product immediately, and not after cleaning, yes.
But azeotrope and sulfuric acid are bought, and nitric acid >90% must be distilled. It's at least a long time. This is justified for a lot of
explosives, where it is simply impossible or unproductive to use azeotropic nitric acid. But this is clearly not a PETN.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
OK I believe I dried my crude ETN sufficiently to get a decent weighing. This time the result was much much better. I received 220.5g out of a
starting 120g erythritol.
So this does give me reason to maybe experiment a little more with ammonium nitrate for for ETN, but not PETN.
| Quote: | | But azeotrope and sulfuric acid are bought, and nitric acid >90% must be distilled. It's at least a long time. This is justified for a lot of
explosives, where it is simply impossible or unproductive to use azeotropic nitric acid. But this is clearly not a PETN. |
I cannot buy any nitric acid, sadly. If I could buy azeotropic nitric acid as cheaply as I could buy drain opener grade sulfuric acid (which can be
purified and concentrated further easily with some H2O2 and boiling), it would be a whole different thing for me, I wouldn't bother with this process,
and making high concentration 90+% HNO3 would be even easier by just distilling it again with more sulfuric acid or magnesium nitrate.
But like I said, I gotta make most things from scratch.
|
|
|
pjig
Hazard to Others
  
Posts: 182
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Quick question on stabilization of the final product. As literature claims, ETN stores poorly. Even if the materials are washed and stabilized with
nitrogen scavenger, how long can this material safely bee stored? I know it’s big brother PETN store's indefinitely once re-crystallized and
purified. I guess if stored properly cool location could years be possible for ETN?
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
I’ve never had problems with ETN stability. If properly neutralized and stabilized it will last years.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
| Pages:
1
2
3 |