| Pages:
1
2
3
4 |
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
Ohh I see, well that would probably be better than using such a large amount of AlCl3 on the Gattermann reaction.
Would a small plug of cork attached to a rod make poking it out of the condenser easier? I was planning on running it and periodically disconnecting
the condenser and ramming that down and collecting the powder in a container then putting it back.
If it clogs too easily I might try having a female-female joiner at the end of the reaction tube and then more copper pipe attached (perhaps I could
cool it with the hose too) so it could be run for much longer, then the second half can be disconnected and scraped out in a similar way. It'd also be
easier to cram more foil back in the tube while it's still hot hopefully.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
If you keep the entire tube hot, you can sublime the AlCl3 out into a receiver at room temperature instead of letting it clog the reaction tube. But
you need something like a glass rod running through a plug of asbestos tape to clear out blockages that do form, no matter what you do.
It might be worth mentioning that some Friedel-Crafts can be run using Al, sometimes Al(Hg) instead of AlCl3.
|
|
|
major_tom3
Harmless

Posts: 1
Registered: 29-8-2010
Member Is Offline
Mood: No Mood
|
|
Guys, please excuse a beginner's naive questions.
Background: I need an anhydrous Lewis acid, say AlCl3 or FeCl3.
1. I want to do a reaction in CH2Cl2 so what I was going to do was:
- buy FeCl3 crystals, readily available for etching printed circuit boards.
- dissolve in dic
- stick in loads of drying agent, say sodium sulphate
- agitate, wait until I get bored
- filter and use
Now I'm guessing this won't work, or you lot wouldn't be playing with chlorine in your back yards.
Can someone point out the flaw in my master plan?
2. Is FeCl3 far inferior to AlCl3?
Thanks in advance.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Ground control to major tom:
Think of the water as an integral part of the molecular structure. When hydrated Al3+ becomes more like [Al(H2O)6]3+
Drying can be done with thionyl chloride which reacts with the water replacing it with Cl
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Given the level 4 rating it scores on the NFPA 704 fire diamond, coupled with it being controlled under the chemical weapons acts, I would recommend
you don't use thionyl chloride.
You can dry FeCl3 hexahydrate (the dark yellow) with heat under a stream of hydrogen chloride gas.
A number of lewis acids behave this way with regards to drying by heat, they start fuming off hydrogen chloride gas as you try to dry them. By running
them under that gas, they don't.
Lewis acids are roughly grouped into hard and soft. The hard and soft groups alone aren't perfect definitions, there are difference between the acids
within those groups in terms of how aggressively they seek out electron pairs.
To produce clean, anhydrous lewis acid by thermally drying the damp veriations, you'll need to produce a clean, dry stream of hydrogen chloride gas.
If you've not done this before, I would suggest practicing first, bubbling it into water to make up some hydrochloric acid. And starting with small
amounts.
The gas isn't systemically toxic, but it's beyond 'a bit reactive' or 'a bit smelly'. It'll rot stainless and chrome fittings, normal keck clips fall
to bits when exposed to very tiny leaks through the tapers, tubing goes 'funny', tubes tend to block up as things react at their ends and so on.
Drip some concentrated sulphuric acid on some table salt in a tiny test tube, then have a deep sniff and see if it's something you want to make a lot
of. Be prepared for a 30 second long, involuntary coughing seizure. 
[Edited on 30-8-2010 by peach]
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
Well put, Peach.  Your description of HCl is spot-on. Your description of HCl is spot-on.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I'd like to carry out a Friedel–Crafts very soon, so I'd like to give this a shot. I don't have a gas grill, instead I have a charcoal grill that
can definitely get up to temperature, I've melted aluminium in it once upon a time, and steel can glow red hot if I force air into it, so temperature
is not a problem. I also have some sodium bisulphate that I can mix with salt to generate HCl.
However, I'm wondering if I can use a glass condenser for re-condensing aluminium chloride vapours. Won't soda lime glass crack under the temperature
gradient? I mean, one side is at 400C+ and the rest is at 20C. Could I use a copper tube instead?
|
|
|
Thor
Harmless

Posts: 19
Registered: 13-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | I'd like to carry out a Friedel–Crafts very soon, so I'd like to give this a shot. I don't have a gas grill, instead I have a charcoal grill that
can definitely get up to temperature, I've melted aluminium in it once upon a time, and steel can glow red hot if I force air into it, so temperature
is not a problem. I also have some sodium bisulphate that I can mix with salt to generate HCl.
However, I'm wondering if I can use a glass condenser for re-condensing aluminium chloride vapours. Won't soda lime glass crack under the temperature
gradient? I mean, one side is at 400C+ and the rest is at 20C. Could I use a copper tube instead? |
I am also planning on trying this soon. The Bisulphate sounds like a nice way to generate dry HCl, although according to wiki, the reaction proceeds
at 200C. I suppose you could do it all in the BBQ  . .
I planned on having the copper tube exiting straight into an old jar surrounded by ice water. Dunno if this will be sufficient though.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
"I am also planning on trying this soon. The Bisulphate sounds like a nice way to generate dry HCl, although according to wiki, the reaction proceeds
at 200C. I suppose you could do it all in the BBQ  . .
I planned on having the copper tube exiting straight into an old jar surrounded by ice water. Dunno if this will be sufficient though."
Sounds like a nice idea, however the jar with the ice water sounds like a failed venture from the start. There's a reason why the OP used room
temperature water. If you use ice water any water that was present in the tube would re-condense and contaminate your anhydrous aluminium chloride.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | There's a reason why the OP used room temperature water. If you use ice water any water that was present in the tube would re-condense
|
Your reasoning is incorrect. If there is water in the tube, it will already have combined with the AlCl3 (which will then not be anhydrous). The use
of rt water is likely just a matter of convenience; if you're condensing something with a bp of 158C then the difference between 0C water and 20C
water is not worth worrying about.
|
|
|
Thor
Harmless

Posts: 19
Registered: 13-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  |
Sounds like a nice idea, however the jar with the ice water sounds like a failed venture from the start. There's a reason why the OP used room
temperature water. If you use ice water any water that was present in the tube would re-condense and contaminate your anhydrous aluminium chloride.
|
At these reaction temperatures, any water in the system will be long gone before the reaction begins. If you where using wet HCl then of course water
would be continually added. But your Bisulphate method does not start producing HCl till 200C. Water will not be a problem.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
To bbartlog & Thor.
I understand. But how were you planning on attaching the jar to the copper tube and putting a vent without letting atmospheric humidity contaminate
the condensed aluminium chloride?
My reasoning is that you need to have some kind of tube between the copper tube and whatever condenser you plan to use (since the temperature gradient
would crack any kind of glass, including boro). Also, you can't attach the jar hermetically because you need some kind of vent for the hydrogen gas
that is evolved. The problem: once the HCl generator stops producing hydrogen chloride, the gases inside the copper tube are still very hot. When the
temperature goes down, the gasses contract, reducing the pressure and causing humid air to get sucked in through the hydrogen vent. Since the jar the
air would be entering into would be at 0C, it's only logical to think that atmospheric water would condense on its sides and contaminate the product.
If air doesn't get sucked in, the tube or the jar would implode, making a mess, and wasting your time.
How would you deal with this problem? The OP has an argon tank, but what would normal people use?
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
If 20degC water was used, very little atmospheric water would condense onto the sides of the jar when air gets sucked in.
|
|
|
Thor
Harmless

Posts: 19
Registered: 13-6-2011
Member Is Offline
Mood: No Mood
|
|
A good point. I was thinking of brazing the tube into the top of a jar, with a metal lid, or even using a compression fitting. A few could be run in
series to stop moisture entering and to maximize capture of the AlCl3. I have a cylinder of argon, but I feel it may be overkill. I also have lots of
glass, but some things are more suited to some metal thrown together.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I was just thinking about using a long, straight copper tube condenser cooled with room temperature water. That way, the AlCl3 condenses, atmospheric
humidity does not, the H2 flows out the other end of the copper tube and no glass in involved. To remove the product, I will take another tube,
slightly thinner than the condenser, and push the crystals out into a vial with desiccant for storage.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | To bbartlog & Thor.
I understand. But how were you planning on attaching the jar to the copper tube and putting a vent without letting atmospheric humidity contaminate
the condensed aluminium chloride?
My reasoning is that you need to have some kind of tube between the copper tube and whatever condenser you plan to use (since the temperature gradient
would crack any kind of glass, including boro). Also, you can't attach the jar hermetically because you need some kind of vent for the hydrogen gas
that is evolved. The problem: once the HCl generator stops producing hydrogen chloride, the gases inside the copper tube are still very hot. When the
temperature goes down, the gasses contract, reducing the pressure and causing humid air to get sucked in through the hydrogen vent. Since the jar the
air would be entering into would be at 0C, it's only logical to think that atmospheric water would condense on its sides and contaminate the product.
If air doesn't get sucked in, the tube or the jar would implode, making a mess, and wasting your time.
How would you deal with this problem? The OP has an argon tank, but what would normal people use?
|
Ah, now I understand your reasoning and it makes sense. However, a drying tube or something similar (at the end, where atmospheric air is going to get
sucked back in) would be more reliable than trying to condense the moisture; in particular 0C is hardly going to get all the moisture out of the air.
Having an exhaust tube stuffed with baked-dry cotton balls is one way.
As for the connection between copper and glass, yes... some sort of luting is needed.
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
Ok so what about storage? Can it be stored in a hot garage? My guess is that it can since it is formed at high tempuratures. It will not decompose in
a hot garage I am sure.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Is this possible to dehydrate Aluminium Hexahydrate by Thionyl Chloride(SOCl2)?
| Quote: |
ANHYDROUS METAL CHLORIDES
MCln.xH2O + xSOCl2 + MCln + xSO2 + 2xHCl
General methods for the preparation of anhydrous chlorides have been
described by Tyree. Of the methods listed for dehydrating metal chlorides,
that involving treatment with thionyl chloride has the advantages of
convenience and simplicity and requires no special apparatus. This method is
generally useful regardless of the periodic group in which the metal appears.
Anhydrous Metal Chlorides
Robert J. Angelici
DOI: 10.1002/9780470132593.ch80
|
[Edited on 9-7-2013 by Waffles SS]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yes, but from the perspective of the amateur chemist, why? It's much easier to make anhydrous AlCl3 than it is to make SOCl2.
Quote: Originally posted by peach  | Given the level 4 rating it scores on the NFPA 704 fire diamond, coupled with it being controlled under the chemical weapons acts, I would recommend
you don't use thionyl chloride.
[Edited on 30-8-2010 by peach] |
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Thought to give a hit for this one.
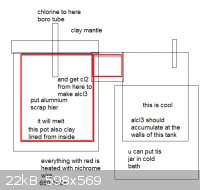
How about this kind of reactor:
Steel body, with ceramic clay inner and nicr heater wire
Quartz or boro tube blowing dry chlorine gas
Melt aluminium at 650-700C
Blow chlorine into it
AlCl3 will form and distill up to tube
The tube is heated too
Tube goes into cooled receiver where alcl3(FIXDED) should accumulate
Any ideas how to effectively prevent clogging the tube? Planning of using at least 3/4" or 1" steel tube.
[Edited on 10-7-2013 by testimento]
[Edited on 10-7-2013 by testimento]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
You're using "nicr" wire, -50 × C, exhaling chlorine, forming "AlCl3," and collecting "al2o3"‽
[Edited on 7/10/13 by bfesser]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
How about a starter project? A propane-powered lime furnace is fairly easy,
and you'll learn about the difference between oxidation and reduction firing. Maybe an electric furnace that can fire red earthenware at cone 2.
You'll learn a lot, at least enough to know that your grand plans are currently beyond you.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Actually, I already obtained the material for both of them, but Im still waiting for the nichrome wire and glass tube. I'll be pleased to post pics of
the process soon when the stuff comes. I've got several other things to do at the moment, including the furnace, so not today. 
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by Cheddite Cheese  | Yes, but from the perspective of the amateur chemist, why? It's much easier to make anhydrous AlCl3 than it is to make SOCl2.
Quote: Originally posted by peach  | Given the level 4 rating it scores on the NFPA 704 fire diamond, coupled with it being controlled under the chemical weapons acts, I would recommend
you don't use thionyl chloride.
[Edited on 30-8-2010 by peach] |
|
I have access to thionyl Chloride,but it seems it just can dehydrate Aluminium Chloride Hexahydrate not Aluminium chlorohydrate(PAC).
Adding aluminium metal directly to thionyl chloride is possible route to anhydrous AlCl3?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I tried to dehydrate Aluminium Chloride Hexahydrate by Thionyl chloride by below instruction.
| Quote: |
General methods for the preparation of anhydrous chlorides have beendescribed by Tyree.' Of the methods listed for dehydrating metal chlorides,that
involving treatment with thionyl chloride2 has the advantages ofconvenience and simplicity and requires no special apparatus. This method isgenerally
useful regardless of the periodic group in which the metal appears.
Anhydrous Metal Chlorides
Robert J. AngeliciAlfred R. Pray1,Richard F. Heitmiller2,Stanley Strycker2,Victor D. Aftandilian2,T. Muniyappan2,D.
Choudhury2,Milton Tamres2
Published Online: 5 JAN 2007
DOI: 10.1002/9780470132593.ch80
|
I used 120gr(AlCl3.6H2O) and 350gr(SOCl2).I have to say nothing happened after adding resulting powder to water.No heat No vapor no gas.
I dont think SOCl2 is effective substance for dehydrating Aluminium Chloride Hexahydrate

|
|
|
| Pages:
1
2
3
4 |