| Pages:
1
2
3 |
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Melgar, I have followed this thread and I can only say that you have excellent observation capabilities combined with the ability to make a connection
with theory. Nicodem has done a nice experiment in a more controlled environment using the right equipment. This is the kind of hobby chemistry I
really like. Keep up the good work. I also consider doing this reaction now, even although I am not really into organics.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thanks.
Sedit, if you want to do a preparative reaction using TCCA, then just follow the literature procedure (the JOC paper cited above and attached in a few
posts already) and thus use benzoyl peroxide as radical initiator. This is the classical Wohl-Ziegler reaction on toluene (interestingly, TCCA was
used for the Wohl-Ziegler reaction already in their seminal paper - the example was allylic chlorination of cyclohexene). If instead of benzoyl
peroxide you want to use sunlight or artificial light to initiate the reaction, then just heat up toluene to reflux and slowly add either very small
portions of TCCA or use an addition funnel to slowly drip in an ethyl acetate solution of TCCA while irradiating with strong (sun)light. This way you
can not have any runaways as you never have too much of the chlorinating reagent present (unless you get stupidly impatient with the addition rate).
Perhaps it is optimal to use an addition funnel with TCCA in ethyl acetate so that while the addition progresses, the reflux temperature lowers down
due to the lower bp of ethyl acetate. Use a large excess of toluene or else you will end up with a thick slurry of cyanuric acid (CA) and furthermore
potentially get some PhCHCl2 and PhCCl3 side products (remember also that the stoichiometry of the reaction is 3PhMe + TCCA => 3PhCH2Cl + CA).
Isolate by filtering off the precipitate, wash it with some toluene (or ethyl acetate or petroleum ether), and then fractionate the filtrate using a
good distillation column.
If you are careful enough you might actually do all this without tears. Be careful especially when dismantling and cleaning the distillation
apparatus. Otherwise, ammonia is good enough to quench benzyl chloride, though it takes quite some time.
Using quat bromides or t-BuOH is hardly of any use in a preparative reaction given the reaction proceeds also in their absence. The only benefit would
be in decreasing the required reaction temperature, for example if one would want to do the reaction at room temperature. But since the reaction is
highly exothermic, it will heat up anyway, so instead of removing heat via reflux condenser you would have to use a cold bath in order to maintain low
temperatures. A few quat bromides are sold OTC as algaecides. Perhaps it would be of use to those who do not have a reflux condenser or any proper
glassware, but otherwise I'm not sure if using any such catalysis has any other special advantage (like I said the chromatograms were identical for
all reaction mixtures exposed to sunlight). This is however of much interest for research, yet from my experiments I am not able to conclude anything
about the catalysis mechanism of TEBAB or of the LiBr catalysed reaction described by Melgar.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Wow, thanks Nicodem! I've been trying to get access to the chem labs at my university so I could get enough data to potentially publish a paper, and
this makes me much more hopeful. Plus, the professors may be more likely to hear me out if there's some (albeit informal) verification of my results.
I have actually done the reaction with TCCA and LiBr once already, but it generated all this white precipitate which I thought was probably cyanuric
acid, but couldn't test to be sure. Thus, I used chlorine gas since it would make the products easier for me to identify. Still, it's great to know
that the reaction can be done just as easily with TCCA.
My only test so far for more chlorinated toluene derivatives was to mix the result with an alcohol solution of NaOH. The benzyl chloride smell
gradually disappeared, but there was no benzaldehyde smell that would have been indicative of the presence of benzal chloride. Since there was benzyl
chloride, but no benzal chloride, I figured it was a safe bet to assume there was also no benzotrichloride.
As far as uses for this reaction, it could probably be used to make grignard reagents. Toluene wouldn't interfere with a Grignard reaction, so it
wouldn't even need to be distilled beforehand. The HCl and chlorine would have to be given time to evaporate though. Although, if Nicodem's process
is used, the cyanuric acid could just be allowed to settle out, thus minimizing the production of noxious gases.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | | If instead of benzoyl peroxide you want to use sunlight or artificial light to initiate the reaction, then just heat up toluene to reflux and slowly
add either very small portions of TCCA or use an addition funnel to slowly drip in an ethyl acetate solution of TCCA while irradiating with strong
(sun)light. This way you can not have any runaways as you never have too much of the chlorinating reagent present (unless you get stupidly impatient
with the addition rate |
I did manage to quell the runaway by slow addition AFTER the PhMe or PhMe2 in other test reached critical temperatures(PhMe2 has a lower runaway
temperature) in the past but unless I read your post wrong you did seem to suggest that the bromine carrier did indeed allow the reaction to proceed
at low temperatures with out the run away is this correct? I was also using impure Ca(OCl)2 as the hypochlorite so next attempts will all be TCCA
since I have sourced it cheeply now and its purer.
I would like a set it and forget it method if you get my drift. Something I would be able to leave out in the sun for a few days with little worry of
runaway or over chlorination. The halogen carrier appears as though it may quell the worrys of exploading bottles of toluene when things get hairy.
I feel IpOH may also work in place of t-BuOH as well when using hypochlorites as the chlorine source and there was a thread a while back discussing
the rapid chlorintion of Toulene using Alkylhypochlorites but I was turned off by the sensitivity to explosion on exposure to light. If they work
catalyticaly in this process then a small amount of IpOH could go along way and avoid the hazards of the infamous "chlorine bombs" You tube is
littered with.
I eagerly away your data on ring chlorination products if any are formed.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Umm - aldehydes react in the presence of strong base. In the case of an aldehyde such as benzaldehyde, with no alpha hydrogens, you get the Cannizzaro
reaction yielding benzyl alcohol and sodium benzoate. If the water content is low enough you could get the Tishchenko reaction, giving the
corresponding ester.
I'd base the presence or absence of polyhalo compouds on the ratio of chlorine to toluene, in your case around 1:10 which with the reactions and
reactants makes polyhalogenation unlikely.
Bubbling CO2 or N2 through the mix would remove HCl, as would adding a toluene solution of a tertiary base or better a tert-base ion exchange resin as
it would be easier to filter off.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Woelen - thanks! I started trying to teach myself chemistry about six months ago. Fascinating stuff! I like how everything we can see is made out
of chemicals, and is capable of any number of reactions. 
Sedit - if my past experience holds true for you, you'll only have to leave your vessel out in the sun for about 15 minutes. 
not_important - I thought the Cannizzaro reaction was really slow at room temperature. Anyway, in that experiment, the ratio was probably higher than
1:10, since I continued chlorinating it after the reaction finished the first time. Nicodem's results seem to confirm that the polychlorinated
products are negligible, since he used more chlorine than I did.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I was left with some 20 mL of the 0.5M TCCA solution in PhMe/EA and since I had no chlorotoluenes standards to verify their presence via HPLC, I added
0.25 mL CF3COOH to catalyse the electrophilic chlorination and left this reaction mixture standing overnight. The next day there was cyanuric acid
precipitate and the HPLC analysis showed that the product(s) formed (presumably o- and p-chlorotoluenes) have the retention time of 7:46 min (there
was only one broad peak which might be o- and p-chlorotoluene overlapping; traces of BnCl could also be seen but that was because I did not wrap the
flask in aluminium foil to protect from light). I went back to check if there is anything at 7:46 min in the chromatograms I made yesterday and there
was nothing obviously rising beyond the background, except for a negligible peak at 8:00 min in the reaction mixture A. So if any chlorotoluenes
formed they formed in negligible amounts only.
1H NMR analysis of the intact reaction mixture (minus the insoluble CA) also confirmed this, as there were no unambiguously identifiable singlets for
the o-chlorotoluene's and p-chlorotoluene's methyl groups (lit. values of the chemical shifts taken from SDBS are 2.34 and 2.29 ppm respectively). The
two singlets that might belong to them were partially overlaping with the PhMe singlet so their integrals are misleading. But even if taken as
they are, there should be less than some 5% of chlorotoluenes in relation to BnCl. No signals for PhCHCl2 could be found at all. (Edit: Actually, I
just realized the tiny singlet at 6.67 ppm is from PhCHCl2. It molar ratio in relation to BnCl is 1 : 100. So the amount of dichlorination is
pretty much negligible when using a threefold excess of toluene.)
The reaction conversion to benzyl chloride was estimated from the BnCl/PhMe ratio which was found to be 1 : 4.3 from 1H NMR spectra. The initial
ratio of reagent vs. substrate was 0.5 mmol TCCA per 0.5 mL toluene (4.7 mmol). According to my calculation, the conversion was about 60%, but
estimating conversion with NMR is not particularly accurate, especially this way and without any internal standard.
An interesting thing observed in the 1H NMR spectra is the set of signals that in my opinion belong to ethyl acetate chlorinated at the alpha ethyl
position: CH3COOCHClCH3. This can only occur via radical chlorination, because the electrophilic chlorination of ethyl acetate could only give ethyl
chloroacetate (ClCH2COOCH2CH3). I should go find the NMR spectral data in the literature to confirm the identity of this side product, but don’t
have the will to do so now. So, for all those interested in the synthesis of acetaldehyde and its derivatives, here you have a potentially new
photochemical route starting from a common solvent.
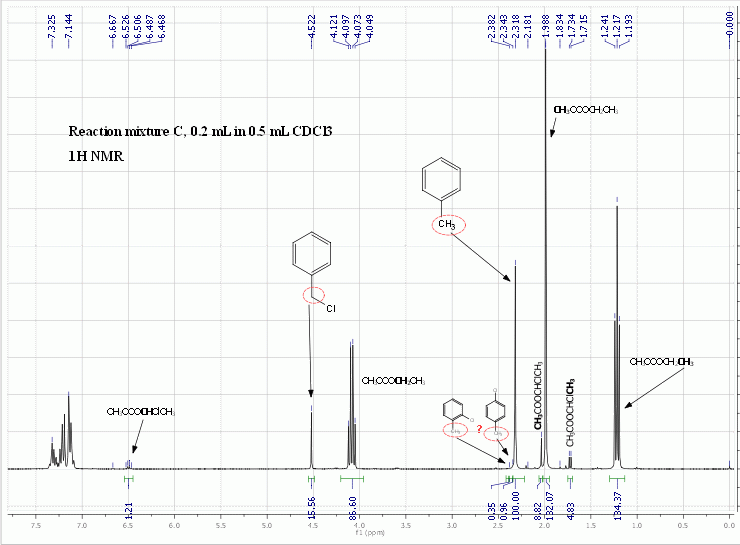
[Edited on 1/7/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | | I did manage to quell the runaway by slow addition AFTER the PhMe or PhMe2 in other test reached critical temperatures(PhMe2 has a lower runaway
temperature) in the past but unless I read your post wrong you did seem to suggest that the bromine carrier did indeed allow the reaction to proceed
at low temperatures with out the run away is this correct? I was also using impure Ca(OCl)2 as the hypochlorite so next attempts will all be TCCA
since I have sourced it cheeply now and its purer. |
What is sold as calcium hypochlorite is actually Ca(OCl)Cl or CaCl2/Ca(OCl)2 in varying proportions. Ca(OCl)2 is not easy to come by. Anyway, "calcium
hypochlorite" in this context is something else altogether when compared to chloroimides. You have a heterogenous mixture of an oxidant and a fuel
that will only react once they reach a certain temperature and once they do they practically blow up. On the other hand, with TCCA you have a way more
controlled media for the Wohl-Ziegler reaction as long as you do it properly.
What you seem to not properly understand is that a reaction has the same reaction enthalpy regardless if it is catalysed or not. The only thing that
changes in the presence of a catalyst is the activation energy. In short, this means, that yes you can have the reaction run at a lower temperature
when using a catalyst, but the amount of heat liberated will be the same as in the one without the catalyst. If you compare my experiments A with C
and D you can see that already on a 2 mmol reaction scale the heat liberated was enough for a boil over in A. The only reason why C and D did not
reach the boiling point is because they started earlier and slowly so that the heat of the reaction had 9 minutes more to dissipate. Now, imagine you
would scale up by 100 times to an amount of 200 mmol TCCA! The surface to volume ratio certainly does not increase by a factor of 100, so in such a
case where you would have a reaction scale of 200 mmol you would have the same runaway regardless of the presence or absence of whatever catalyst. You
simply need to remove the heat of the reaction!
| Quote: | | I would like a set it and forget it method if you get my drift. Something I would be able to leave out in the sun for a few days with little worry of
runaway or over chlorination. The halogen carrier appears as though it may quell the worrys of exploading bottles of toluene when things get hairy.
|
No, you would still get an exploding bottle, unless you would be able to inversely regulate the light exposure with the reaction temperature. In no
case don't you just put it on direct sunlight! You keep forgetting that you are dealing with a highly exothermic radical chain reaction. These
reaction can not be upscaled without severe modifications or run safely unless you have a slow addition of the limiting reagent. You can't just mix
them together on a large scale, furthermore concentrated, and then lit with a radical initiator / light. Radical chain reactions don't have stable
kinetics because the rate of the reaction depends not only on the activation energy, but is terribly dependent on the length of radical chain
propagation. This very strongly depends on many conditions: temperature, light intensity, radical initiator concentration and properties, and even
some things you can not control in any way or are pretty much unknown. So is it strange that they end up with a runaway if you just "set it and forget
it"?
That's why the safest way to do it is by using Cl2. You limit the reaction by the chlorine gas inflow and don't have to worry about such things as
flasks blowing up with lachrymogenic benzyl chloride.
| Quote: | | I feel IpOH may also work in place of t-BuOH as well when using hypochlorites as the chlorine source and there was a thread a while back discussing
the rapid chlorintion of Toulene using Alkylhypochlorites but I was turned off by the sensitivity to explosion on exposure to light. If they work
catalyticaly in this process then a small amount of IpOH could go along way and avoid the hazards of the infamous "chlorine bombs" You tube is
littered with. |
iPrOH might work but it would soon die out as it gets oxidized rapidly under the reaction conditions (forming acetone and HCl). I would avoid it. Why
don't you try with just any tetraalkylammonium bromide? As far as I know, some are available OTC. Or just buy some tetrabutylammonium bromide (TBAB).
It is cheap and you might need it for other experiments as well. But like I said, don't live in the illusion that any such additive will prevent a
runaway if you premix all the reagents and expose them to sunlight. You need to use some way to remove the reaction heat, either via the reflux
condenser in an "non-catalysed" reaction, or via a cold bath using a "catalyst". But in any case always have TCCA as the reaction limiting reagent to
be added slowly.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
kmno4
International Hazard
    
Posts: 1496
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | This is however of much interest for research, yet from my experiments I am not able to conclude anything about the catalysis mechanism of TEBAB or of
the LiBr catalysed reaction described by Melgar. |
I do not believe this 
Formation of BrCl (from Br2, formed from ionic bromide and Cl2) is rather obvious and it looks like Br radical is only chain initator. Something more
about radical reactions Cl2/Br2/BrCl can be found here.
However, it seems that it is not "real" catalyst because it should be consumed during reaction.
[Edited on 1-7-2010 by kmno4]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I had to research proton NMR before I could understand that last post, but now that I get most of it, it's quite fascinating. I am curious as to why
you used ethyl acetate? Also curious as to how you'd use this reaction to get acetaldehyde. Personally, I'm partial to using chlorine gas, that way
you can't have too much of the limiting reagent (since only so much chlorine will dissolve in toluene) and there's nothing to separate out afterwards.
I did notice a mist forming above my vial that I believe was the gas-phase version of this reaction whereby chlorine is radicalized by the action of
UV light. I'm not sure if this would happen with TCCA, but if so, that could be where the benzal chloride is coming from. Or perhaps it just forms
in small quantities anyway.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I made bromo toluene in the way described, and reacted with AgNO3 in toluene to make hydroxy,nitrotoluene ( and some traces of PhCH2ONO2).
I think all the organic people are missing out by not checking my reactions in the Energetic section of this forum.
Check out "explosives from quinone". All the commentators there seem more interested in dangerous acids and explosions, than the actual organic
chemistry required to make compounds that have any more complexity than basic TNT or nitroglycerine.
[Edited on 2-7-2010 by Anders Hoveland]
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
You mean AgNO2? Or is this some novel process of your invention?
[Edited on 2-7-2010 by Satan]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I probably got methyl,hydoxy, nitro benzene. Where the hydroxy could be anywhere in relation the methyl group, and the nitro group is adjacent to
either the hydroxy, or the methyl group. Obviously this creates several combination possibilities. Phenyl nitrate is unstable. Toluene nitrate would
also be if the nitrate was no on the methyl.
I did not use silver nitrite. There are no nitronium ions that form either.
[Edited on 2-7-2010 by Anders Hoveland]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | Quote: Originally posted by Nicodem  | | This is however of much interest for research, yet from my experiments I am not able to conclude anything about the catalysis mechanism of TEBAB or of
the LiBr catalysed reaction described by Melgar. |
I do not believe this 
Formation of BrCl (from Br2, formed from ionic bromide and Cl2) is rather obvious and it looks like Br radical is only chain initator. Something more
about radical reactions Cl2/Br2/BrCl can be found here.
However, it seems that it is not "real" catalyst because it should be consumed during reaction. |
Nice find! I was already afraid nobody would take the time to check the literature for anything related to the topic.
BTW, I did not say I can not come up with a hypothesis (I can always do that for just about anything, but that is cheap  ), just that I'm "not able to conclude anything" (that is to prove any such
hypothesis based on those experiments alone). One surprising observation from the NMR is that there is no BnBr formed (well, there is "something" at
4.44 ppm where PhCH2Br should be, but that something could be anything and it integrates for even less than the PhCHCl2 peak). If all the bromide from TEBAB got
consumed into benzylic bromination, there should be some more BnBr formed (theoretically 1.6% in relation to BnCl, which is a bit too little for NMR
and calls for a GC analysis). So, for some reason the intermediate species that allows the reaction to smoothly run already at room temperature is
either highly selective for chlorination (like BrCl or more?) or something else happens. On the other hand it appears as if TEBAB is either consumed
or made insoluble (note that not all dissolved!) as its cation can not be detected either (its NMR is also available at SDBS). So, there are still questions to be answered before giving a conclusion. One obvious thing that should be done
is to try using some quat bromide that would completely dissolve and in a higher amount so to see if there is some BnBr formed or whatever is the fate
of the bromide. ), just that I'm "not able to conclude anything" (that is to prove any such
hypothesis based on those experiments alone). One surprising observation from the NMR is that there is no BnBr formed (well, there is "something" at
4.44 ppm where PhCH2Br should be, but that something could be anything and it integrates for even less than the PhCHCl2 peak). If all the bromide from TEBAB got
consumed into benzylic bromination, there should be some more BnBr formed (theoretically 1.6% in relation to BnCl, which is a bit too little for NMR
and calls for a GC analysis). So, for some reason the intermediate species that allows the reaction to smoothly run already at room temperature is
either highly selective for chlorination (like BrCl or more?) or something else happens. On the other hand it appears as if TEBAB is either consumed
or made insoluble (note that not all dissolved!) as its cation can not be detected either (its NMR is also available at SDBS). So, there are still questions to be answered before giving a conclusion. One obvious thing that should be done
is to try using some quat bromide that would completely dissolve and in a higher amount so to see if there is some BnBr formed or whatever is the fate
of the bromide.
Quote: Originally posted by Melgar  | | I had to research proton NMR before I could understand that last post, but now that I get most of it, it's quite fascinating. |
You better read about NMR as much as you can, because if you will ever choose organic chemistry, this analytical method will become your daily
routine. About 20% of the time in the lab (or more) is spent running NMR scans and interpreting spectra. It is annoying but you can't do much without
it. Chromatographic methods only give you products separation, but without standards they can not be used for their identification. It is the
spectroscopic methods which give the answer, and single crystal XRD the one that gives the final answer (if needed).
| Quote: | | I am curious as to why you used ethyl acetate? Also curious as to how you'd use this reaction to get acetaldehyde. |
TCCA does not dissolve very well in plain toluene, so I wanted to use a cosolvent to get a homogeneous 0.5M solution. If you want to draw
conclusions from experiments and compare different conditions, it is best if the reaction mixtures are homogeneous. It is also important to know the
amount of reactants and that is why I could not use Cl2. Of course, for a preparative reaction it does not matter if TCCA has poor solubility in
toluene, because you always can and must use stirring to compensate. I had only few choices of which cosolvent to use: acetonitrile, ethyl acetate or
acetone, as these are the ones which dissolve TCCA very well and do not easily react with it. Alcohols get O-chlorinated by TCCA and the so formed
hypochlorite esters are extremely photolabile (see experiment with t-BuOH added!). Standard polar aprotic solvents like DMF, DMSO or NMP react with
TCCA (DMSO extremely rapidly and exothermically) while inert chlorinated solvents like CH2Cl2 do not dissolve TCCA any better than toluene. Acetone
was out of play because it is the more reactive than MeCN or EtOAc toward TCCA, and because I was afraid it would quench the radical chain
propagation. For some reason I expected MeCN is more prone toward radical chlorination than EtOAc so I chose this later (luckily I was wrong ). In fact there are very few reports of such radical chlorination of ethyl acetate
in the literature and such a synthesis of MeCOOCHClMe is very attractive. This compound easily hydrolyses to acetaldehyde and it can also be used as a
masked acetaldehyde (a vinylidene synthon) in several reactions where acetaldehyde or paraldehyde is otherwise used. This means that the radical
chlorination of ethyl acetate with TCCA gives an excellent opportunity to prepare this useful reagent that might be used in several interesting
reactions or as a starting material for the preparation of acetaldehyde (hydrolysis), acetyl chloride (retro-acylation), vinylidene acetals
(alcoholysis) or vinyl acetate (elimination). It also opens a new potential route to aldehydes starting from esters. Several other compounds could
also be radically chlorinated this way to give useful compounds. Ethers are already known to be very easily alpha-chlorinated by TCCA, but many other
compounds could be as well, perhaps even acetonitrile to give chloroacetonitrile and so on. ). In fact there are very few reports of such radical chlorination of ethyl acetate
in the literature and such a synthesis of MeCOOCHClMe is very attractive. This compound easily hydrolyses to acetaldehyde and it can also be used as a
masked acetaldehyde (a vinylidene synthon) in several reactions where acetaldehyde or paraldehyde is otherwise used. This means that the radical
chlorination of ethyl acetate with TCCA gives an excellent opportunity to prepare this useful reagent that might be used in several interesting
reactions or as a starting material for the preparation of acetaldehyde (hydrolysis), acetyl chloride (retro-acylation), vinylidene acetals
(alcoholysis) or vinyl acetate (elimination). It also opens a new potential route to aldehydes starting from esters. Several other compounds could
also be radically chlorinated this way to give useful compounds. Ethers are already known to be very easily alpha-chlorinated by TCCA, but many other
compounds could be as well, perhaps even acetonitrile to give chloroacetonitrile and so on.
| Quote: | | Personally, I'm partial to using chlorine gas, that way you can't have too much of the limiting reagent (since only so much chlorine will dissolve in
toluene) and there's nothing to separate out afterwards. |
Yes, like I explained to Sedit, if you are unsure what you are doing, it is best to use chlorine on larger scale preparative reactions. Though,
generating large amounts of chlorine is also hazardous, but again only if you don't know what you are doing. The bottom line is, that in every case
you should know what you are doing and here lies the problem. I have read here about people designing experiments on a large scale (why?) without even
doing a literature search first, let alone thinking of what can go wrong, so I'm afraid someone will do something stupid again.
| Quote: | | I did notice a mist forming above my vial that I believe was the gas-phase version of this reaction whereby chlorine is radicalized by the action of
UV light. I'm not sure if this would happen with TCCA, but if so, that could be where the benzal chloride is coming from. Or perhaps it just forms
in small quantities anyway. |
Where the benzylic proton of benzylidene chloride should be (at 6.695 ppm according to SDBS) there is almost nothing as you can see above, so only trace amount of PhCHCl2 formed. But you have to keep in mind that I used
a threefold excess of toluene and that TCCA could be more selective toward monochlorination than Cl2.
[Edited on 2/7/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Check out this paper:
http://dx.doi.org/10.1016/0039-9140(58)80019-3
Apparently, BrCl acts exclusively as a brominator. I suspect this reaction produces only chlorinated versions of organobromides that are formed via
free-radical halogenation. Thus, the bromine forms radicals, brominates the hydrocarbon, and is replaced by chlorine, which forms more BrCl. For
example:
RH + Br* + Cl* -> RBr + HCl
RBr + Cl2 -> RCl + BrCl
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
@ Nicodem
Forgive me if this sounds like shameless sycophancy but I'm seriously convinced that you direct some pharma company's research division. Your posts
set a standard for what should be reported on this board.
I agree with kmno4 that you must look for bromide present in the product via GC; that would settle whether or not bromide had a catalytic role.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
While we all can't quite achieve such a level of awesome, those who could and can sure do make an awesome contribution to our knowledge. Thanks
Nicodem.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
hey i have a question for you guys seeing how i'm dumber than a box of rocks, i was curious, if one were to do the same chlorination on a toulene with
a ring activating group meta to the benzylic carbon for instance 2-hydroxy toulene for example.
would this increase the likelihood of side reactions such as ring halogenation to any great extent?
seeing how the ring is a bit more activated and there is greater electron density locant to the benzylic carbon would this also increase the
possibility of dichlorination to the benzal chloride?
excuse my stupidity i'm just looking to get spoonfeed.
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
I will chime in my appreciation for Nic's experimentalism.
I am still on about the topic of styrene. What would be the result of free-radical chlorination of styrene with TCCA? I note that there is no Cl- ion
produced in chlorinations w/ TCCA since all Cl atoms are in the +1 state, so a vacinal chloride would not form under anhydrous conditions, but this
could be inconsequential to the addition of chlorine at all. Maybe the chlorine would add to the β carbon first forming chlorostyrene, then
additional additions on either the α or β carbon forming 1,2 dichlorostyrene or 2,2 dichlorostyrene.
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by jon  | hey i have a question for you guys seeing how i'm dumber than a box of rocks, i was curious, if one were to do the same chlorination on a toulene with
a ring activating group meta to the benzylic carbon for instance 2-hydroxy toulene for example.
would this increase the likelihood of side reactions such as ring halogenation to any great extent?
seeing how the ring is a bit more activated and there is greater electron density locant to the benzylic carbon would this also increase the
possibility of dichlorination to the benzal chloride?
excuse my stupidity i'm just looking to get spoonfeed. |
Cresols do not undergo free radical chlorination easily. 30 seconds with google turned up at least one patent for a workaround chlorination of cresol
esters: http://www.google.com/patents/about?id=E4guAAAAEBAJ.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | I am still on about the topic of styrene. What would be the result of free-radical chlorination of styrene with TCCA? I note that there is no Cl- ion
produced in chlorinations w/ TCCA since all Cl atoms are in the +1 state, so a vacinal chloride would not form under anhydrous conditions, but this
could be inconsequential to the addition of chlorine at all. Maybe the chlorine would add to the β carbon first forming chlorostyrene, then
additional additions on either the α or β carbon forming 1,2 dichlorostyrene or 2,2 dichlorostyrene. |
TCCA is too electrophilic for radical halogenation of styrene (is radical chlorination of styrene even possible?), or styrene is too nucleophilic for
TCCA, depends on the perspective. It is the same problem as in trying to radically chlorinate cresols. In principle electrophilic chlorination of
styrene with chloroimides should give beta-chlorostyrene, but in practice and with CH2Cl2 as solvent, the reaction of styrene and TCCA results in
styrene oligomerization. The oligomerization is most likely electrophilic: in the sense that the initially formed PhCH<sup>+</sup>-CH2Cl
adds on a styrene and so on until carbocations dye out by H<sup>+</sup> elimination. But I was never that interested to run a GC-MS on the
product mixture to see if the oligomers start with PhCH=CH- and end with -CH2Cl groups. I only did a few analyses to see if my desired product was
there as the major product or not - oligomers don't interest me (though I remember that they nicely separated on TLC, so they could be isolated). But
this was years ago when I still worked on halogenations and I do not remember much of the details.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, free radicals tend to cause styrene to polymerize. Plus, the evolved HCl can add to alkenes.
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by manimal  | | I am still on about the topic of styrene. What would be the result of free-radical chlorination of styrene with TCCA? I note that there is no Cl- ion
produced in chlorinations w/ TCCA since all Cl atoms are in the +1 state, so a vacinal chloride would not form under anhydrous conditions, but this
could be inconsequential to the addition of chlorine at all. Maybe the chlorine would add to the β carbon first forming chlorostyrene, then
additional additions on either the α or β carbon forming 1,2 dichlorostyrene or 2,2 dichlorostyrene. |
TCCA is too electrophilic for radical halogenation of styrene (is radical chlorination of styrene even possible?), or styrene is too nucleophilic for
TCCA, depends on the perspective. It is the same problem as in trying to radically chlorinate cresols. In principle electrophilic chlorination of
styrene with chloroimides should give beta-chlorostyrene, but in practice and with CH2Cl2 as solvent, the reaction of styrene and TCCA results in
styrene oligomerization. The oligomerization is most likely electrophilic: in the sense that the initially formed PhCH<sup>+</sup>-CH2Cl
adds on a styrene and so on until carbocations dye out by H<sup>+</sup> elimination. But I was never that interested to run a GC-MS on the
product mixture to see if the oligomers start with PhCH=CH- and end with -CH2Cl groups. I only did a few analyses to see if my desired product was
there as the major product or not - oligomers don't interest me (though I remember that they nicely separated on TLC, so they could be isolated). But
this was years ago when I still worked on halogenations and I do not remember much of the details. |
That makes sense, since both polymerization and chain chlorination are radical-promoted.
US2803675 (http://www.google.com/patents/about?id=6VpoAAAAEBAJ) makes use of in-situ chlorine generated from HCl and a chlorine-furnishing substance to
side-chain chlorinate styrene in an inert solvent. I imagine it works by using a highly acidic(excess HCl) aqueous phase that ensures that HClO+HCl
<--> Cl2+H2O stays far to the right therefore minimizing the amount of HClO floating around that might hydroxychlorinate or ring chlorinate the
styrene.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I did not say that styrene polymerized. I said it oligomerized and I did not say it was a radical promoted oligomerization. I don't really remember
much as this was a long time ago (relative to my poor memory capabilities at least) and I don't know where my lab diary of that period is or on which
backup CD did I put the spectroscopic data, but the major oligomers were of n<7 or thereabout, which is more consistent with the carbocationic
polymerization. I took care of preventing any radical polymerization (low temperature, absence of light, highly electrophilic source of "positive
chlorine", etc.). I do not know what would happen and what would be the major products in the radical chlorination of styrene as radical halogenations
were never part of my research or interest. I just described what happened in my attempt in reacting styrene with TCCA under the described conditions
(my goal was an efficient synthesis of beta-chlorostyrene). I would assume that for an efficient radical chlorination of styrene the temperature would
have to be high enough for the polymerization to be reversible enough and radical chlorination not (I would guess something like about 200 °C or
more!), else the major product would be terminally chlorinated polystyrenes and not much else. However, I'm not really able to conceive a reagent
being non-electrophilic at such conditions, yet being a source of Cl* radicals (perhaps CCl4?). But since I do not know enough about radical
halogenations, don't take my words too seriously.
Edit: Quickly checked the patent you linked. That is not a radical chlorination of styrenes! It just "normal" electrophilic chlorination. The
electrophilic addition of Cl2 to styrenes gives 1-aryl-1,2-dichloroethanes and the electrophilic atack followed by the deprotonation of the chloronium
ion gives the other type of products, beta-chlorostyrenes. And yes, the biphasic system prevents the formation of the halohydrins and the presence of
HCl keeps the concentration of HClO and other oxychloro species low (as it reduces them).
[Edited on 27/8/2010 by Nicodem]
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | I did not say that styrene polymerized. I said it oligomerized and I did not say it was a radical promoted oligomerization. I don't really remember
much as this was a long time ago (relative to my poor memory capabilities at least) and I don't know where my lab diary of that period is or on which
backup CD did I put the spectroscopic data, but the major oligomers were of n<7 or thereabout, which is more consistent with the carbocationic
polymerization. I took care of preventing any radical polymerization (low temperature, absence of light, highly electrophilic source of "positive
chlorine", etc.). I do not know what would happen and what would be the major products in the radical chlorination of styrene as radical halogenations
were never part of my research or interest. I just described what happened in my attempt in reacting styrene with TCCA under the described conditions
(my goal was an efficient synthesis of beta-chlorostyrene). I would assume that for an efficient radical chlorination of styrene the temperature would
have to be high enough for the polymerization to be reversible enough and radical chlorination not (I would guess something like about 200 °C or
more!), else the major product would be terminally chlorinated polystyrenes and not much else. However, I'm not really able to conceive a reagent
being non-electrophilic at such conditions, yet being a source of Cl* radicals (perhaps CCl4?). But since I do not know enough about radical
halogenations, don't take my words too seriously.
Edit: Quickly checked the patent you linked. That is not a radical chlorination of styrenes! It just "normal" electrophilic chlorination. The
electrophilic addition of Cl2 to styrenes gives 1-aryl-1,2-dichloroethanes and the electrophilic atack followed by the deprotonation of the chloronium
ion gives the other type of products, beta-chlorostyrenes. And yes, the biphasic system prevents the formation of the halohydrins and the presence of
HCl keeps the concentration of HClO and other oxychloro species low (as it reduces them).
|
OK, Nic.
What do you suppose is the point of the solvent in the "normal" chlorination of styrene with Cl? Most procedures use CCl4 or similar inerts, unlike in
chlorinations of toluene.
|
|
|
| Pages:
1
2
3 |