| Pages:
1
2
3 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | The words asymmetric and symmetric are very unfortunate in that context and should be replaced by chiral and achiral. For example levo- and
dextrotartaric acid are symmetric by twofold rotation but are definitely chiral. Chirality is the absence of improper rotations (i.e. operations with
det(r)=-1) but says nothing on proper rotations. Same thing for crystals (e.g. quartz which has trigonal symmetry but is chiral). Also "chiral centre"
is problematic, but you know all that of course...  |
The words asymmetric and symmetric were referring to the reaction and not to the structural symmetry as you misunderstood. I was trying to explain the
stereochemical "dogma" that a reaction can give an asymmetric outcome (excess of one enantiomer over the other) only in an asymmetric starting
environment. Structures can be chiral or achiral, but reactions proceed symmetrically or asymmetrically. But I do agree that "chiral centre" is a
problematic phrase. As a student I too was forced to read Eliel's bible on stereochemistry and should know better, but old habits die hard.
Quote: Originally posted by turd  |
The first step is intriguing. I would have thought that the methylamine would be removed with the water phase. Does this work for phenylacetones...?
 |
Aldehydes (like benzaldehyde is) form way more stable imines than ketones do. In fact many aldehydes will form stable imines or their trimeric forms
(hexahydro-s-triazines) even in water. Therefore I doubt you could do the same with a ketone using the same treatment. Ketones often require
the use of SnCl4 to form enamines/imines and that is quite some harsh reagent.
Still does not beat the simplicity of the Stevens rearrangement! 
I can't imagine anything more simple using just two steps and three simple reagents, though it is true that it ends up with the racemic mixture.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
As a student I too was forced to read Eliel's bible on stereochemistry and should know better, but old habits die hard.
|
Oh gosh I remember having to read that! What a nightmare! Perhaps I should revise it, I have finals this year :|
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | I can't imagine anything more simple using just two steps and three simple reagents, though it is true that it ends up with the racemic mixture.
|
Photoamination of stilbene? Could it be the great day I have surpassed the master?!!! Hehe, only joking 
Edit - No it isn't! Only ammonia and primary amines from what I have seen... But here is the ammonia paper anyway.
Attachment: Photoamination of Stilbenes.pdf (546kB)
This file has been downloaded 1063 times
[Edited on 19-1-2010 by sonogashira]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | The words asymmetric and symmetric were referring to the reaction and not to the structural symmetry as you misunderstood. I was trying to explain the
stereochemical "dogma" that a reaction can give an asymmetric outcome (excess of one enantiomer over the other) only in an asymmetric starting
environment. Structures can be chiral or achiral, but reactions proceed symmetrically or asymmetrically. But I do agree that "chiral centre" is a
problematic phrase. As a student I too was forced to read Eliel's bible on stereochemistry and should know better, but old habits die hard.
|
Point taken, even though symmetric reaction is of course a fuzzy notion. A gas-phase chemist will think of a symmetric reaction when it consumes as
many molecules as it generates. And to be fair, you also applied the term symmetric to the reactants and the reaction medium, when IMHO it would make
more sense to call them chiral or with an enantiomeric excess. But since this is a forum and not a textbook we're not going to be holier than the
pope.
Speaking of textbooks, I never heard of Eliel and probably never will look inside since this book probably has a rather high hair-loss factor. Indeed,
it took me a long time to come up with the example of tartaric acid, while quartz was immediately evident.
Quote: Originally posted by Nicodem  | | Aldehydes (like benzaldehyde is) form way more stable imines than ketones do. In fact many aldehydes will form stable imines or their trimeric forms
(hexahydro-s-triazines) even in water. Therefore I doubt you could do the same with a ketone using the same treatment. Ketones often require
the use of SnCl4 to form enamines/imines and that is quite some harsh reagent. |
Excuse me while I go into a corner and cry for a while...  On Rhodium's archive
there is a procedure by LabTop who makes the phenylacetone n-methylimine with silica gel, but I'm not sure if it's not a little bit dubious. Of course
I will not lose bad words on a procedure until I've tried it - a project for a future rainy day... On Rhodium's archive
there is a procedure by LabTop who makes the phenylacetone n-methylimine with silica gel, but I'm not sure if it's not a little bit dubious. Of course
I will not lose bad words on a procedure until I've tried it - a project for a future rainy day...
Back on topic: I think the original poster got all the needed pieces and I probably speak for everyone when I say: we want results! Maybe not to the
final product, but N,N-dimethylbenzylamine and benzylbromide would be nice.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I used Labtop's method for phenylbutanones and methyl and dimethylamine, workep faily well, although I didn't use any silicagel, just anhydrous amine
in MeOH, stirred for an hour at 10°C, then added NaBH4 in portions over a few hours. Steam distilled the amines, got 60-75% yields.
I also used the same technique with benzaldehdye although I realise now I could ahev used 40% aq. MeNH2 instead of going through the trouble of
gassing MeOH...
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It can be, but would you give some reference ?
Not about Stevens rearrangement at all but for this particular case. I could not find anything about it (use of KNH2 and 30% yield is not worth
mentioning)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
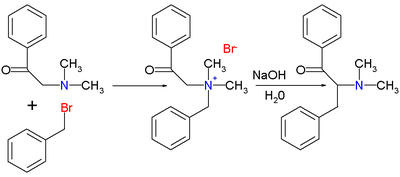
Only 30% with NaNH2? Too bad, but then who uses NaNH2 any more. However, the yield using tBuOK in refluxing toluene is quite good. The quat can be
prepared by refluxing the mixture of BnNMe2 and BnCl in toluene before adding the base. Needles to say, BnNMe2 can be prepared from HNMe2 and BnCl or
PhCHO by the known methods. The reaction works for some other Bn2R2NX where R is alkyl or part of an cycloaliphatic ring.
I will not post the exact reference because I don't want to play the spoonfeed game of the original poster, however it is in a certain RSC journal in
one of the seminal papers from Stevens et al. where they study this rearrangement. The competing reaction is the Sommelet-Houser rearrangement. The
ratio between the products of the two types of rearrangement is very dependent upon the base used. The best results were obtained by
tBuOK>iPrOK>>MeOK if I remember well, MeOK giving almost exclusively the Sommelet-Houser rearrangement product.
Then there is also a paper by Wittig et al. in Ann. from 1951 where they form the ylide from Bn2Me2NBr by using PhLi and analyse the decomposition
products (amine ylides decompose rapidly). The yield of lefetamine by this decomposition is 52% while the Sommelet-Houser rearrangement product
(N,N-dimethyl-alpha-(o-tolyl)benzylamine) is 36%. The separation was done simply via differences in solubility of the hydrochlorides.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | (cut)The words asymmetric and symmetric were referring to the reaction and not to the structural symmetry as you misunderstood. I was trying to
explain the stereochemical "dogma" that a reaction can give an asymmetric outcome (excess of one enantiomer over the other) only in an asymmetric
starting environment. Structures can be chiral or achiral, but reactions proceed symmetrically or asymmetrically. But I do agree that "chiral centre"
is a problematic phrase. As a student I too was forced to read Eliel's bible on stereochemistry and should know better, but old habits die hard. (cut)
|
Does anyone know of a free download of Stereochemistry Of Organic Compounds, by E L Eliel (Wiley-1994)? I cannot find one.
However, using Google, I found another book edited by him, Topics In Stereochemistry:
http://rs358tl3.rapidshare.com/files/94358058/topics_in_ster... 14.6 Mb
As regards asymmetry in organic reactions, this thesis is relevant:
http://www.biorg.uu.se/Forskning/BLa/TorbenR/thesis_pub.pdf - Molecular Modeling Of Asymmetric Catalysis (2001) 907 Kb
[Edited on 21-1-10 by JohnWW]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Topics in Stereochemistry, Volume 9
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 08:45
Topics in Stereochemistry, Volume 4
by: Ernest L. Eliel, Norman L. Allinger
en | Wiley
2008-11-22 07:40
Topics in Stereochemistry, Volume 5
by: Ernest L. Eliel, Norman L. Allinger
en | Wiley
2008-11-22 08:00
Topics in Stereochemistry, Volume 8
by: Ernest L. Eliel, Norman L. Allinger
en | Wiley
2008-11-22 08:33
Topics in Stereochemistry, Volume 10
by: Ernest L. Eliel, Norman L. Allinger
en | Wiley
2008-11-22 08:52
Topics in Stereochemistry, Volume 1
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 07:01
Topics in Stereochemistry, Volume 2
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 07:23
Topics in Stereochemistry, Volume 3
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 07:34
Topics in Stereochemistry, Volume 6
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 08:09
Topics in Stereochemistry, Volume 7
by: Norman L. Allinger, Ernest L. Eliel
en | Wiley
2008-11-22 08:23
.............this may be of interest, found here.....
http://gigapedia.info/1/E.L.%20Eliel
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
In have found it in part VII... but with no yield given. Procedure is not so simple as it looks.
Nevermind.
I gave 30% earlier for KNH2 in refluxing toluene , but it is my mistake and yield is 59%(+37% recovery of input compound)). In liquid NH3 there is 97%
yield of another product (ortho substitution).
This paper is cited on given earlier webpage.
BTW I think that more a propos literature is "The chemistry of enamines" (from Patai series). Available on internet.
|
|
|
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
I apologize for doing such a terrible job creating my original post. i did several hours of research (yes not enough) before posting. i felt that i
had looked into the different methods of synthesis and the amount of information i posted was much more than any other forum post or paper i could
find. im sorry for not including sources. menawhile thank you everyone for the information and the great conversation. please understand im not asking
to be spoon fed, i was asking for exactly what we have here, a wonderful discussion on the compound.
its been several years since O chem and i admit i love the lab work much more than the theory and for that reason i am very nervous about saying
anything intelligent for fear that i will be wrong ;/
so back to the topic and again im sorry. This is a shot in the dark but its an idea based on the very beautiful Stevens arrangement posted earlier.
According to production of bronchodialator effects patent #3089862 from 1958 o-chlorophenethylamine reacted with 37% formic acid, 90% formaldehyde at
reflux will produce 0-chloro-N,N-dimethylphenethylamine. so as of yet i have not found (i am looking) any direct evidence that this will work but what
do you think of:
phenylethylamine + formic acid + formaldehyde ===> N,N-dimethylphenethylamine
 + +  + +  =======> =======> 
 + +  ==NaOH===> ==NaOH===>
This would then be reacted with Bromobenzene. then according to papers ive seen on the stevens arrangment NaOH is used to shift the carbon bond from
the nitrogen onto the carbon chain. walla! is there any reason that bromobenzene would not work when replaced for benzyl bromide?
bromo benzene can be made rather easily if one has benzene and a brominating agent (br2 right?). PEA is very easy to get in pure form from the corner
health food store even. formic acid and formaldehyde are rather common as well and the formic acid can be made. If you cant get a hold of NaOH, give
up now  .references if requested .references if requested
From there it would be a separation of enantomers. i have yet to find anything with tartaric acid but i will continue to look.
I understand that this was somewhat already mentioned but the specific precursors were not mentioned.
thanks for the input everyone. and again im sorry if im asking for to much. i feel that this post asks for some help but also gives some mildly new
information as well.
|
|
|
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
sorry for the double post but i found another reference for phenethylamine to n,n-dimethylphenethylamine.
http://www3.interscience.wiley.com/journal/113342277/abstrac...
and
http://www.freepatentsonline.com/y2005/0033088.html
not exactly what i was hoping for but very close.
[Edited on 21-1-2010 by kafka]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes, but I'm not going to spoonfeed you. Go and find out why.
[Edited on 21-1-2010 by DJF90]
|
|
|
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
so bromobenzene will not work with n,n-dimethylphenelethylamine for making lefetaimne correct? before i spend a good amount of time finding out why i
want to make sure you dont think i ment bromobenzene for producing the product shown in the scheme that i copied and placed in my post for the
stevensrearrangment.
we would want the carbon chain to be one carbon shorter than whats shown. the example given is one two long for lefetamine which i hoped to fix by
using bromobenzene wich is one shorter than benzyl bromide. regardless ill go look into the mechanism some more.
im really trying to contribute here and discuss the chemistry not simply ask how to do it.
meanwhile how about :
Toluene + Cl2 ===> benzyl chloride
Toluene + Br2 (add MnO2?) = benzyl bromide
or ammonium bromide, toluene, h202, acetic acid
dimethylamine + benzyl chloride ===> n,n-dimethylbenzylamine
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/b...
n,n-dimethylbenzylamine + benzyl bromide = product
again i dont see how this couldnt work but i am a noob as you all have so easily noticed. the oxygen on the reagent in the first part of the reaction
in the scheme above really doesnt seem to come into play in the mechanism so im hoping leaving it out is a ok.
the great thing about these precursors is that they are all very easy to make. more citations upon request.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Before attempting (and likely failing) to undertake this sizable task, why dont you start with the basics? You need a good solid theory to be able to
suggest any kind of reasonable synthesis, to know what will and won't work. There are many books recommended here for such purpose, and then you can
actually understand the chemistry involved.
I am also disturbed by your continual reference to the easy obtention of supposed precursors. Whilst us chemists should always be resourceful, I would
not appreciate you getting valuable sources of chemicals shut down/removed because you try making a scheduled substance with them and get busted by
the feds.
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
I'm interested in some pretty intense stuff, and thus, I'M LEARNING THE BASICS.
And it's fun.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
i understand this is over my head but please instead of insulting me lets criticize my ideas or add to the thread.
really im trying to do my research before i post.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You misunderstand Kafka; There is no point criticising your ideas until you come up with plausible ones. And you won't be able to do that until you
LEARN SOME CHEMISTRY. So get to it!
The fact you appreciate that this is over your head, yet you are still reluctant to learn the basics, makes me think that you don't CARE about the
chemistry involved and you just want a "theoretical synthesis" to make an "adequequate amount" of Lefetamine, or something of "greater or equal
value". You still did not explain what you meant by this...
You say you wanted a challenge, yet this is too much for you because you have little basic understanding. Set the bar a little lower and have
another stab at it, then you can progress to something more complex like Lefetamine.
Sykes' "A guidebook to mechanistic chemistry" is a very good place to start, as it gives you a good foundation in mechanistic reasoning that you need
as an organic chemist. Theres also a book by Suarez which is "200 exercises in mechanistic organic chemistry" - 2002, which you can then use to TEST
your understanding. For stereochemistry I think the best book is Eliel's "Stereochemistry of organic compounds", and for general organic chemistry the
textbook by Clayden et al. "Organic chemistry" is fantastic.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Please, you're not ready to come up with your own reactions, so you will have to use reactions exactly as they're published (which means you'll have
to learn to use a university library!). Don't try to be clever. Really, just don't try and follow published procedures, otherwise you will only end up
frustrated. For the example at hand: have you not noticed that
1) in all examples on the rearrangement there was either a carbonyl or an aryl functionality next to the C.
2) the posters above are talking about strong bases.
? Even someone who is oblivious of organic chemistry (like me) would conclude that the C probably needs to be acidic.
N,N-dimethylbenzylamine is probably better made by reductive amination of benzaldehyde (bitter almond essential oil, you need it for cooking  ). You will only learn by doing, so go ahead or let it be. ). You will only learn by doing, so go ahead or let it be.
| Quote: | | I am also disturbed by your continual reference to the easy obtention of supposed precursors. Whilst us chemists should always be resourceful, I would
not appreciate you getting valuable sources of chemicals shut down/removed because you try making a scheduled substance with them and get busted by
the feds. |
Come on, come back to reality. Chemicals getting removed from the market because a single amateur tries to make an obscure psychoactive? Absurd.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I have uploaded Sykes' book on my premium Rapidshare account, and there is a link for it in the Organic Books in the References section.
As for the book by G T Suarez, Google yields several free downloads, including:
http://rushim.ru/books/mechanizms/two-hundred-exercises-in-m... 1.25 Mb
and
http://rs41l33.rapidshare.com/files/268200770/Two_hundred_ex... 507 Kb
[Edited on 21-1-10 by JohnWW]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Chemicals getting removed from the market because a single amateur tries to make an obscure psychoactive? Absurd. |
Hardly. I worked for a chemical supplier a long time ago and we would get a call from some whacked out freak asking for something
that we didn't know was a precursor. The company immediately stopped selling the stuff. I have to wonder where kewls get these strange ideas that
the chemical industry is drug friendly.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Even if your anecdotal evidence is true (you'll get the benefit of the doubt), this only proves that there are companies with silly business
practices. The competition will rejoice.
My personal anecdotal evidence indicates the opposite of your conclusion: As a whacked out freak, kewl or whatever you wish to call me, I have never
had problems buying potential precursors to various street drugs (and really, what isn't?). The various companies generally seem to have a "don't ask"
policy, which is of course sane, since they want to keep their customers and customers value privacy. Only once I had to confirm with a signature that
I will not use an oxidizer for making bombs. *shrug*
But aside from anecdotal evidence we could simply have a look at the facts:
para-formaldehyde and ammonium chloride, precursors to methylamine, in turn a key precursor to methamphetamine and MDMA: easily available.
benzaldehyde: well known precursor to amphetamine: easily available.
benzylbromide: well known precursor to (meth)amphetamine: easily available.
etc, etc, etc...
Or lets have a look at your beloved DEA list I chemicals (http://en.wikipedia.org/wiki/DEA_list_of_chemicals): Without exception, all listed chemicals are directly tied to the multi-kg production of major
street drugs. Not a single one of them was scheduled because of some obscure route to an obscure psychoactive. Sorry to tell you, but reality
completely contradicts your dream world.
PS: didn't you plan to refrain from posting off-topic stuff about your favourite subject?
Please discuss this kind of things in legal or whimsy.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
This looks interesting.
Attachment: ja01215a018.pdf (233kB)
This file has been downloaded 1168 times
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I hope those yields convince you to take a different route. 
|
|
|
| Pages:
1
2
3 |