| Pages:
1
2 |
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by Sedit  | | I see mentioned in this thread the suggestion of adding the HNO3 to the hot glycol, could you explain what advantage this would have?
|
Uh, you know...that part about the runaway? A certain amount of fuming is reasonable but... And maybe although HNO3/V2O5 gives oxalic acid with sugar
and cellulose, it seems unlikely to have unlimited resistance to hot nitric acid. I don't know what the actual result would be since I haven't done
that, but it's an avenue I'd pursue if I had that runaway.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Ok I got it now thanks...
After much thought last night, on the semi scaled up version I ran, and using the means of HNO3 generation that I do(H2SO4+KNO3), I have concluded
that adding the acid to the alcohol to be the best means as well.
Runaways aside the alcohol precipitates dissolved sulfate generating a slush and copious amounts of NOx fumes more dense then I have ever personally
seen. I would like to stir the reaction as it proceeds and the precipitated sulfates would make that all but impossible. Im going to also investigate
some electrochemical means of HNO3 synthesis today since that could possibly make this a very cheep route to AcOH if I can find a means to an end.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I just got a brief bit of time to skim the papers kindly provided by solo and there appears to be a means of adding the Alcohol to the acid while
still avoiding runaways. It appears the initiation of the reaction comes from the presences of NO2 ions and the addition of NaNO2 in proper
concentrations avoids the induction period before the reaction begins meaning that as the alcohol is stirred into the acid it will be turned into the
carboxylic acid avoiding the runaway reaction.
This will allow me to keep a huge excess of oxident present without the prior fears of a runaway. The most interesting part is that the oxidation of
EtOH and IpOH both result in the desired product of AcOH.
[Edited on 23-6-2010 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
OK I know im talking to myself here but I would love to hear some input on my new take on this reaction. I know im deviating abit from the thread
topic but the reaction remains the same.
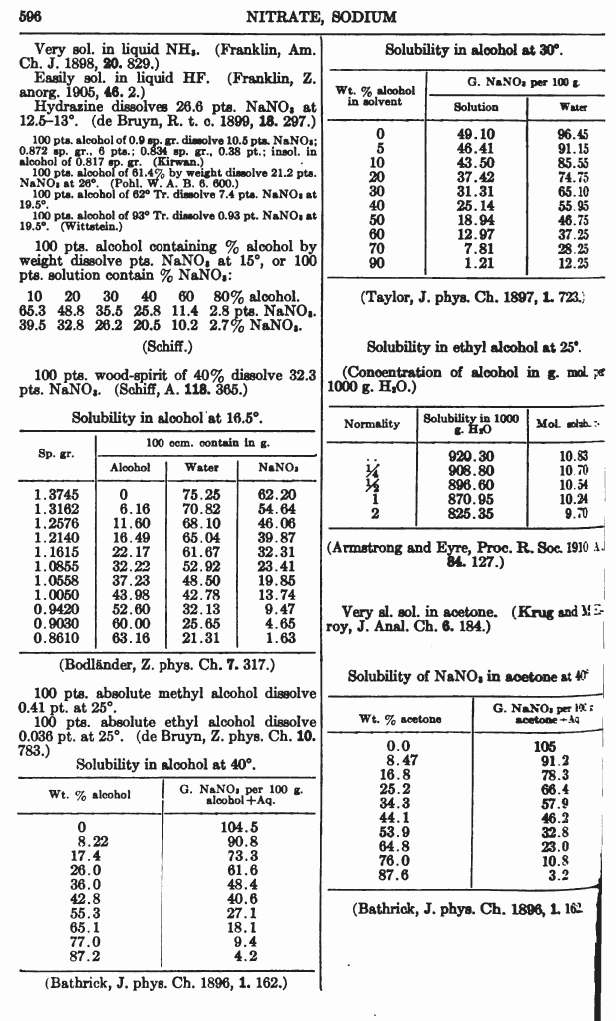
Sodium nitrate is soluble in EtOH, something dont have the full solubility data on just yet.
What about adding H2SO4 to a solution of NaNO3 in EtOH. HNO3 is formed on the spot and it could quell the dreaded runaway by generating localized
heating leading to "completion" of the reaction before it has a chance to take off. This is been suggested in small scale already but I only have
clean KNO3 right now and its solubility in alcohol isn't that great yet its showing success better then other means.
Can anyone forsee problems doing it this way that im missing? Im thinking with stong stirring and ice cooling this should proceed smooth as a babys
behind followed with a post reaction heating to push it to total completion.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
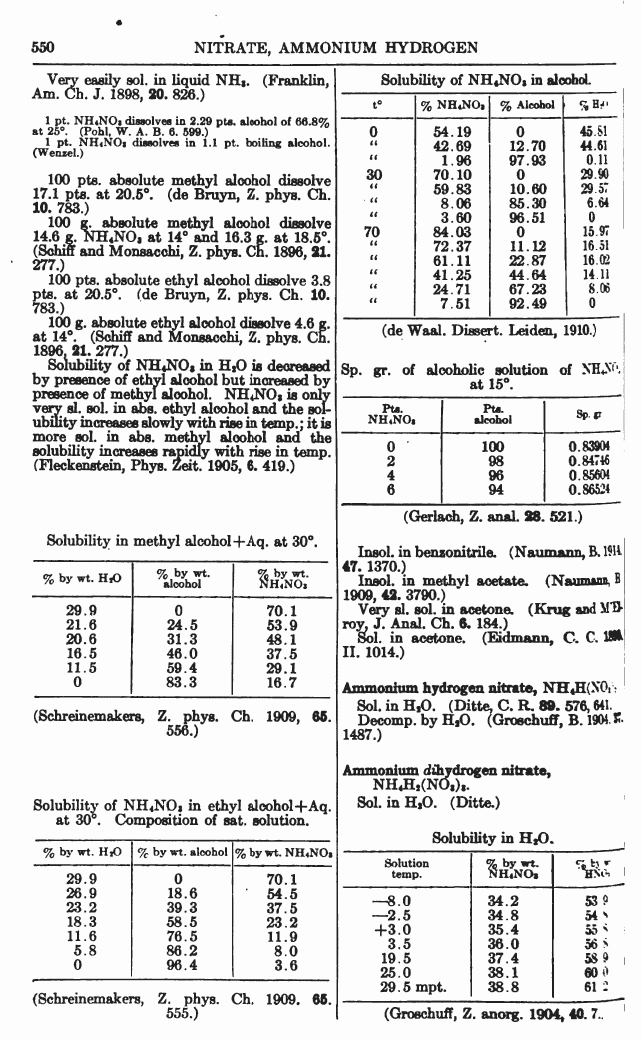
From
A Dictionary of Chemical Solubilities, Inorganic 2nd Ed. Comey
Available at the Internet Archive and Google Books
(and yes, the calcium nitrate file is just CaNO3... because Ca(NO3)2... has some potential filesystem squibble.
I don't see your reasoning matching what would happen, I think that a runaway is even more probable. Also, given the near anhydrous conditions I'd
expect EtONO2 to form.
If nothing else I'd dissolve the H2SO4 in some EtOH first, so as to avoid thermal input from it reacting with the alcohol in the nitrate mix. More
heating drives the reaction faster, that's what the runaway is about, the heat of the reactions speeding up the reactions even more.
(remember - babies bottoms may be smooth, but consider the high volume of you-know-what that comes out of them :-)
Personally, I don't see the reason to waste expensive or increasingly difficult to obtain oxidisers on simple lower alcohols; I'm a bit puzzled as to
why you've chosen this route.
 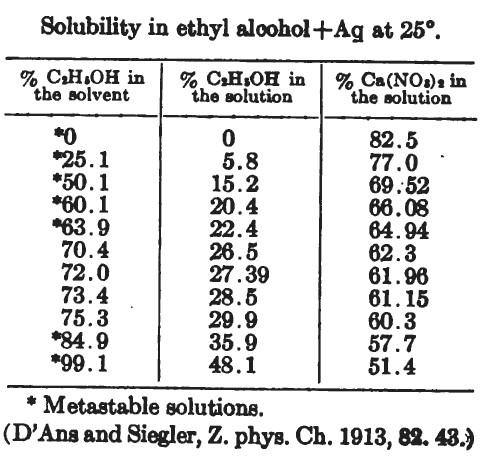 
[Edited on 25-6-2010 by not_important]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
http://www.ab.ust.hk/hseo/tips/ls/ls005.htm
http://users.wpi.edu/~rajat/MSDS%20Ethanol.pdf (Read section 5)
Sounds like a bad idea to me.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by not_important  | From
I don't see your reasoning matching what would happen, I think that a runaway is even more probable. Also, given the near anhydrous conditions I'd
expect EtONO2 to form.
If nothing else I'd dissolve the H2SO4 in some EtOH first, so as to avoid thermal input from it reacting with the alcohol in the nitrate mix. More
heating drives the reaction faster, that's what the runaway is about, the heat of the reactions speeding up the reactions even more.
Personally, I don't see the reason to waste expensive or increasingly difficult to obtain oxidisers on simple lower alcohols; I'm a bit puzzled as to
why you've chosen this route.
[Edited on 25-6-2010 by not_important] |
Thank you for the solubility data I owe you one.
To answer last question KNO3 and H2SO4 are accessible and cheep for me and given the prices discussed and researched on large amounts of AcOH I am
confident I can produce this chemical cheeper then buying it from a supplier perhaps around half price or less.
Dissolution of the H2SO4 into EtOH has crossed my mind as well simular to how I performed the EtBr synthesis sometime back. This is the next step in
my small scale before moving on BTW and has been on the checklist for sometime.
Now back to the most important question.
""I don't see your reasoning matching what would happen, I think that a runaway is even more probable. Also, given the near anhydrous conditions I'd
expect EtONO2 to form."""
Reasoning is as such....
Small scale experimentation has shown what I have assumed. Heating occures in a local area causing the "runaway" in a very local area of the drip of
acid quickly. Stirring cools this back to "baseline" with ease since the higher temperatures are only in a local area. The runaway so to speak has
shown considerable larger concentrations of AcOH over acetaldahyde then allowing the reactants to mix then react "slowly" over time.
The Ester IS my main concern since it will result in a large loss of yeild and adding H2O is no issue since I have decided the best workup might be to
distill, dry, and extract with DCM. I have had success of extracting (aq) solutions of AcOH so this part is a no brainer for me once the AcOH is
synthesized.
I desire this reaction because after years of research this appears to be the cleanest and cheepest means of producing large amounts of GAA
economically. The NaOAc + H2SO4 method sucks and produces impure product. This is showing MUCH more potential then that by far.
Lambda, Nitration is much more hazardest then this reaction yet everyone seems to have no issue running that daily. This is not going to sit on the
shelf for anytime so the dangers associated are null.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by Sedit  |
Lambda, Nitration is much more hazardest then this reaction yet everyone seems to have no issue running that daily. This is not going to sit on the
shelf for anytime so the dangers associated are null. |
I misinterpreted your post. My bad. My understanding was that you didn't want the nitric acid and the ethanol to react.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
When you say | Quote: | | The NaOAc + H2SO4 method sucks and produces impure product. |
do you mean the vinegar as the source of
acetate, or any source of NaAc?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Vinegar of course. Im sure Pure NaOAc would produce a better product but no matter how clean I try to get it the results still appear tainted.
Perhaps its due to decomposition products or poor recrystalization methods(something I pride myself in BTW) but in the end its not a good means to
large GAA source. 50-100ml at best is the limit of that reaction before it starts to present issues and im sure there are others here that will
confirm this for me.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
OK
A) I found that using s slow cooker to evaporate the neutralised vinegar until you've got essentially liquid NaOAc.3H2O, then slowly pouring that
into chill i-Pr alcohol with good stirring, gives a pretty clean precipitate of the acetate, partially dehydrated to the monohydrate. Washing the ppt
with several small amounts of further chilled IPA removes much more of the colouring; after a single wash with IPA, adding a bit of water the the
acetate and going through the melt-evaporate-pour and washing the precipitate twice gave me a white product without much loss (the trihydrate's
solubility in EtOH is around 50g/l, in IPA it's lower) A dichloromethane extraction of the powdered solid acetate also might give good results. In
either case sugars in the vinegar would remain with the organic solvent insoluble portion, but as they're non-volatile the distillation of the AcOH
should take care of them.
I'd also consider the oxidation of EtOH by NaOCl catalysed by nickel salts (which form NiOOH/NiO2). Filtering removes the nickel, evaporation leaves
NaCl and NaOAc.3H2O
In either case I believe it is important to avoid localised overheating when adding the strong acid to the NaOAc. Dispersing the acetate in acetic
acid and slowing adding H2SO4 seems to work, a similar approach is used for formic acid when the dehydration to carbon monoxide can be a real
problem.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I found that using an old stainless steel deep fryer that I modified for steam generation to be the best means of concentrating the NaOAc since it has
ability to boil off gallons of liquid in a matter of minutes instead of hours. I will have to give IpOH a shot if I ever go that means again but even
though im a huge kitchen chemist even this seemed a little excessive for 500 grams or so of NaOAc. Ill take electrochemical means that produces NaOAc
from EtOH over evaporation of that much liquid again but thats a method of the acetic acid threads.
I had a chance to attempt the HNO3 oxidation at a one more scale of acid which I sort of botched but showed some interesting results to me anyway. I
used 1:1 KNO3-H2SO4 with 20ml of H2O to increase the fluidness of the acid when I could have gotten away with 1:0.5 nitrate to acid ratio but was not
thinking. However after reading abstract after abstract over the past few days I decided I was going to start with the addition mol of EtOH the paper
recommends of .2 mol or something on the order of around 10 grams of EtOH and slowly add more until NO2 stops being released. After getting all the
way to 50 grams of EtOH and still showing signes of NO2 I stopped because this does not make much sense to me.
The reaction contains the initial NO2 ions needed for initiation of the reaction from over heating as the H2SO4 is added presumably. Drop by drop I
started to add the EtOH to warm acid and stand back to observe. At first I thought stirring would be good but this causes heavy foaming and I found if
I just leave it as is it starts a relatively mild cycle that was not hard to control with addition rate at all. I would still take much caution for
the first few grams of addition since it does show potential for going out of hand. A cold water bath was used for cooling and I think Ice would be
much better since alot of heat is generated at the start of this reaction and with NO2 already present it does not need an initial heat source to get
it going.
One drip of EtOH is enough to cause all of the NO2 to vanish instantly from the flask leading me to think the NO2 ester plays an important role in the
oxidation of some kind.
Even after breaching 1 mol of EtOH there is still signs of NO2 but much calmer then before and the entire reaction is now fluid with the Potassium
bisulfate almost completely dissolved. This would precipitate at the start and I attribute this to the sulfates solubility in AcOH and insolubility in
EtOH where as it could also be in solution from H2O formed in the reaction, that is yet to be seen.
The smell of AcOH(Dont sniff the FLASK drip it on blotter paper) is very very strong now after a slow warm water bath that lasted a couple days since
I was allowing it to react then add a little more and so on....
Its non flammable by any means at this point.
Why would this reaction still show signs of NO2 generation even after 1 mol of EtOH? I hypothesized that I may be able to do about .5 mol but not 1.
Could the acidic nature of KHSO3 be affecting the reaction somehow?
I wish I had a GC or some means of analysing stuff like the big boys do but its hard here down on the bottom. Still betcha I have more fun doing what
I do for free then they do for money
I obviously have alot of work to do before I could give a definitive yeild. I am going to add a few drops to Sodium bicarbonate and see how wet it
stays after its done reacting. NaOAc should be relatively dry where as H2O and EtOH would leave it damp afterwards. And it will allow me to smell for
the presence of EtOH, AcO, and any esters that may form. Freezing the reaction mixture would be a good idea as well if it can indeed freeze. I want to
have a good idea whats in the mixture before I distill it because all this talk of EtOH and Nitric acid forming explosive mixtures has got me a little
uneasy.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Dripping it onto Sodium bicarbonate produced alot of CO2 some of which could be from HNO2 or HNO3 but in the end when it was neutral there was
evidence of alot of presumably H2O remaining, since it contained very little if any of a smell, which is pretty much unacceptable to me. I was unaware
this reaction produced a mol of H2O as it proceeded and this is counter productive to what I wanted.
The liquid remaining also had a remaining smell of something I can not explain that smelled sort of like burnt AcOH but I can not quite explain it.
Surely not the sweet smell of an ester like I would expect. It was only faint though so whatever it was there is not alot of it or it has a weak
reaction to the nose. Going to try to freeze it and see what I get from that test. I hoped the sulfate would suck most H2O out of the reaction but I
don't believe it did. I still non the lest did not detect even a hint of EtOH or AcO smell in the remaining liquid.
Still I know AcOH is produced in a decent quantity(Maybe almost quantatively but I will need to remove all other acids before titrating to determine
the yeild) and I will try to work it up and try to determine yeilds for the sake of science but in the end I think im going to start attempting some
vapor phase reactions to yeild AcOH in the near future but that is another topic for GAA threads and not this one.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Where did all the stuff go???
I tried to repeat Julius B. Cohen's method for oxidising sucrose to oxalic acid with conc. nitric acid and V2O5 but with remarkably little success so
far.
I don't have any conc. HNO3, only 38 w% (6N), so had to adapt the recipe. I ended up with a hybrid between Cohen's and Magpie's (first post in this
thread), figuring that sucrose could very loosely be described as a hexamer of ethylene glycol.
Cohen calls for 20 g sugar (sucrose), 140 ml conc. HNO3 and 0.1 g of V2O5.
I cooked up 10 g, sugar, 140 ml 38 % HNO3 and 0.05 g of V2O5, by adding the sugar to a hot (steam bath) mixture of the nitric and the catalyst. Based
on Magpie's first attempt, this is somewhat sub-stoichiometric in HNO3.
Ready for a run away, instead the solution turned green (V(H2O) +3) first and slowly started fuming NO2. That fuming build up until thick oodles of
brown smoke bellowed out of the flask but without any run away: the solution was boiling away nicely.
After an hour or so on the steam bath the reaction started slowing down and the boiling stopped. I transferred the liquid to a Pyrex jug and started
to heat with a small direct flame, for about another hour, topping up the liquid with water so that the level didn't fall below 100 ml. At the end the
fuming had all but stopped and I reduced the green solution to about 40 ml, cooled and even chilled it but no crystals of HOOC-COOH where forth
coming.
The next day the solution was carefully reduced to almost nothing and a reddish precipitate formed: hydrated V2O5? But little else was found. I
filtered the solution, the filtrate ran green. But adding concentrated Ca2+ yielded no calcium oxalate...
I tied again, this time 5 g sugar, 115 ml 38 % HNO3 + pinch of V2O5. Assuming sucrose is about (EG)6, this is then stoichiometric in HNO3. The
procedure above was used.
This time, after careful and complete evaporation, an amber-reddish crystalline mass was obtained probably less than one gram though. This dissolved
effortlessly in water, turning clear green in the process. Added anh. CaCl2, this dissolved effortlessly, without any calcium oxalate forming!
Am I overheating this stuff and oxidising also the oxalic acid, or what is going on here??? 
One more attempt will be made with 5 g sucrose, 115 ml 38 % HNO3, no V2O5 and cooling on a cold water after the reaction starts...
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
If the oxidation is going too far, then maybe more sugar or less acid would do the trick. Also, adding the acid mixture to boiling sugar solution
will stop the acid being in excess.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Paddywhacker  | If the oxidation is going too far, then maybe more sugar or less acid
would do the trick. Also, adding the acid mixture to boiling sugar solution will stop the acid being in excess. |
Cohen actually recommends adding the sugar to the acid. I can't really see it make much difference but I could be wrong on that. I believe an excess
of nitric is better than not enough but it's more an article of faith than anything else...
Third attempt: this time a mixture of 5 g sugar and 115 ml of 38 % HNO3 (no V2O5) was gradually heated on the steam bath. It took decidedly longer for
the reaction to start but once started, it's indistinguishable from a reaction that includes the catalyst. Interesting to note is that the solution
also turned green, as in the presence of V2O5.
Taking the bottle off the steam bath and the reaction stopped. I see little point in doing that, so continued with the steam bath for about 2 hours,
until NO2 elution had subsided much. That was then left to stand, quasi-stoppered, overnight.
The flask, with camera shake, just after taking off the steam bath. It was then returned to it:

The 100 or so ml of liquid left was transferred to Pyrex receptacle and simmered under partial reflux. The oxidation reaction started up again and
continued for about an hour or so, with the solution also picking up a brownish colour. At about 50 ml it was transferred to a smaller Pyrex
receptacle and gently boiled in to almost nothing. It dried, showing plenty material, including some black stuff. It seems that right to the end some
oxidisable saccharide remains, which then carbonises at the end.
The Pyrex jug, after gentle boiling (with camera shake): the head space is still full of NO2:

After the smoke has cleared: whitish/yellow mass with a black spot. White material can also be seen on the inverted glass lid (right):

To the solid material was then added 10 ml of water: most of it dissolves but not all. It was heated on steam bath for some time, then filtered. The
filtrate is clear but brownish. On the filter: a white powder. The powder was isolated. It doesn't dissolve in water but it does dissolve in an excess
of strong HCl: an oxalate? It will be neutralised with NH4OH and checked for oxalate.
The filtrate was gently evaporated on steam bath until almost nothing, then left to dry: needle like structures so typical of oxalic acid developed
(hard to see in the photo below). Unfortunately the brown muck did not separate out much and the amount of material is very small. Rough yield to be
determined yet but it seems to me that the oxidation of ethylene glycol with diluted nitric (Magpie and other sources) works well, but not the
oxidation of sucrose for which much stronger nitric is needed for more quantitative yields...

I wonder if improvement could be obtained by carrying out the whole operation on steam bath, from reaction to evaporation of water...
[Edited on 25-7-2010 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, a fourth attempt did work: this time 200 ml HNO3 38 % and 8 g of sugar, a ratio I believe uses a slight excess of HNO3.
Most of the reaction was carried out on steam bath, then paused overnight and finished off by gentle simmering. The last 100 ml or so were evaporated
on steam bath. NOx continued to elute up to the last moment.
5 - 6 g of crude oxalic acid, slightly wet and very slightly yellowish:

The crude has been worked up with three precise recrystallisations.
So it's possible to make oxalic acid even from sugar with fairly dilute HNO3. But it will work faster and better with azeotropic or higher HNO3...
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
You can make formaldehyde from it too, at least according to these Authors (they use Lead Dioxide and acetic acid, forming the tetraacetate in-situ
quam temere in nosmet legem sancimus iniquam
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by blogfast25  | | The powder was isolated. It doesn't dissolve in water but it does dissolve in an excess of strong HCl: an oxalate? It will be neutralised with NH4OH
and checked for oxalate. |
In Beilstein it's said that oxalic acid stubbornly retains alkalis, method for purification given there recrystallizes from boiling 10-15% HCl. Then
washing with a little cold water and recrystallizing from alcohol.
[Edited on 31-7-2010 by Formatik]
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Funny, oxalic acid is one of the few I can purchase no questions asked. They use it for a huge number of reasons, look for it via google "oxalic acid
msds" normally works for me.
quam temere in nosmet legem sancimus iniquam
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Formatik  |
In Beilstein it's said that oxalic acid stubbornly retains alkalis, method for purification given there recrystallizes from boiling 10-15% HCl. Then
washing with a little cold water and recrystallizing from alcohol.
[Edited on 31-7-2010 by Formatik] |
Nice tip. But I don't see where the alkali would come from in my case...
Quote: Originally posted by un0me2  | | Funny, oxalic acid is one of the few I can purchase no questions asked. They use it for a huge number of reasons, look for it via google "oxalic acid
msds" normally works for me. |
I actually now found it on eBay...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Having gone a little 'calorimeter mad' of recently I've attempted to measure the approx. enthalpy of reaction for the oxidation of ethanol (a model
for mono alcohols) in watery medium with dichromate (K2Cr2O7) and sulphuric acid. We know these oxidation reactions are highly exothermic: KMnO4 and
glycerine mixtures for instance heat up enough to ignite a Classic Thermite mix.
I came across a classroom recipe for the demonstration of the oxidation reaction of ethanol with dichromate and adapted it very slightly to:
4 ml of 50 % H2SO4
25 ml of water
3 g of K2Cr2O7
to which after mixing, 4 ml of alcohol is then added. This reagent mixture is non-stoichiometric: molar ratio of alcohol to dichromate being about
6:1, instead of the stoichio 3:1. This should guarantee that all dichromate (about 0.01 mol) is reacted and about 0.03 mol of ethanoic acid is formed.
A dry run showed that despite not all the dichromate dissolving in the H2SO4 solution, the reaction starts promptly and runs to completion with all
the dichromate being used up and a hot, non-turbid, deep violet (Cr3+) mixture of excess alcohol, excess acid, ethanoic acid, and potassium and Cr
[+III] sulphates results in a matter of seconds. End temperature was about 70 C, a ΔT of about 50 C: for 0.03 mol ethanoic acid formed that's
very promising.
I then repeated the experiment au calorimeter with precisely measured quantities, including a precisely weighed amount of dry K2Cr2O7. I need
to specify that for 'ethanol', read 'denaturated alcohol', so a mix of mostly ethanol and some methanol was used. For the calculation of reaction
heat, it was assumed that all liquids had the heat capacity of water. To make this more realistic, a 100 ml water of precisely known temperature was
added after the reaction had completed in the calorimeter and the final enthalpy content was then determined after stabilisation..
Thus an estimated enthalpy of reaction for the oxidation of ethanol with K2Cr2O7 of -245 kJ / mol of acid formed was obtained.
That's quite a whopper for a reaction that involves no lattice energies whatsoever!
Of course this is only one data point and the oxidation heat of an alcohol is likely to depend somewhat on the R group (in R-CH2-OH) as well as the
oxidant used but if we assume the oxidation of 1,2 propane diol to malonic acid with nitric acid as attempted here:
http://www.sciencemadness.org/talk/viewthread.php?tid=12832#...
by Magpie to have a comparable enthalpy per mol of oxidised alcohol group, then the oxidation of 0.2 mol 1,2 PD x 2 mol -OH/mol of 1,2 PD x 245/mol of
-OH group = 98 kJ, or 98,000 J.
Assume the 169 ml of 6 M nitric to have the same heat capacity as 169 ml of water, then the temperature increase upon full oxidation of the 1,2 PD to
malonic acid is expected to be ΔT ≈ 98,000 / (4.1813 x 169) =138 C !! No wonder the experiment resulted in painting Magpie's fume hood a
new shade of 'nitric acid'!
So, boys and girls, be careful with these oxidations of diols and triols and make sure you've plenty heat sinks/cooling in place to prevent run aways!
Update - calculation error alert!
Doh! Where I described the stoichoimeteric ratio of alcohol/dichromate to be 3, that should really have been 1.5 as the oxidation of ethanol
to ethanoic acid involves 4 e- (and not 2 e- as previously assumed for some reason):
CH3-CH2-OH + 5 H2O ---> CH3-COOH + 4 H3O+ + 4 e-
The estimated enthalpy of reaction for the oxidation of ethanol with K2Cr2O7 then becomes - 490 kJ / mol of acid formed (and not
-245) and the estimated temperature rise in Magpie's run away, 276 C.
My bad.
[Edited on 2-8-2010 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The value of - 490 kJ/mol sounding so high, I decided to replicate the experiment above, this time using KMnO4 as oxidiser. A dry run showed that the
reduction goes from Mn [+VII] to Mn [+IV], as brown MnO2 precipitates. The assumed stoichiometry here is:
4 MnO4- + 3 C2H6O (ethanol) + 4 H3O+ ---> 4 MnO2 + 3 C2H4O2 (ethanoic acid) + 9 H2O
The recipe used in calorimeter was aimed at oxidising 0.015 mol alcohol to acid, using a limiting amount of precisely weighed KMnO4 and an excess of
alcohol and acid:
4 ml 50 % H2SO4
25 ml water
3.16 g KMnO4
to which after mixing and temperature measurement, 4 ml of alcohol (denaturated) was added. After reaction another 100 ml of water at known
temperature was added and final enthalpy content of the flask determined.
This yielded a reaction enthalpy of - 490 kJ/mol of formed acid. Precisely the same value as above. I think this shows the nature of
the oxidant plays only a minor role, as long as it's powerful enough, of course...
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by not_important  | OK
A) I found that using s slow cooker to evaporate the neutralised vinegar until you've got essentially liquid NaOAc.3H2O, then slowly pouring that
into chill i-Pr alcohol with good stirring, gives a pretty clean precipitate of the acetate, partially dehydrated to the monohydrate. Washing the ppt
with several small amounts of further chilled IPA removes much more of the colouring; after a single wash with IPA, adding a bit of water the the
acetate and going through the melt-evaporate-pour and washing the precipitate twice gave me a white product without much loss (the trihydrate's
solubility in EtOH is around 50g/l, in IPA it's lower) A dichloromethane extraction of the powdered solid acetate also might give good results. In
either case sugars in the vinegar would remain with the organic solvent insoluble portion, but as they're non-volatile the distillation of the AcOH
should take care of them.
I'd also consider the oxidation of EtOH by NaOCl catalysed by nickel salts (which form NiOOH/NiO2). Filtering removes the nickel, evaporation leaves
NaCl and NaOAc.3H2O
In either case I believe it is important to avoid localised overheating when adding the strong acid to the NaOAc. Dispersing the acetate in acetic
acid and slowing adding H2SO4 seems to work, a similar approach is used for formic acid when the dehydration to carbon monoxide can be a real
problem.
|
Surely sugars are only present in malt,brown and balsamic vinegars, shouldn't all white vinegar be distilled?
|
|
|
| Pages:
1
2 |