| Pages:
1
2
3
4
5 |
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Why am I not surprised that the Department of Redundancy is drying and distilling pure, dry solvents?
I've used both flavors of HEET straight out of the bottle for many purposes, after a few preps where I compared them to reagent grade solvents. I
think they are probably pure enough for most work. The isopropoxide prep is fairly demanding in terms of water content and it worked like a charm.
I just hope Sedit's news about "improved" formulations doesn't come to pass.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by entropy51  | Why am I not surprised that the Department of Redundancy is drying and distilling pure, dry solvents?
I've used both flavors of HEET straight out of the bottle for many purposes, after a few preps where I compared them to reagent grade solvents. I
think they are probably pure enough for most work. The isopropoxide prep is fairly demanding in terms of water content and it worked like a charm.
I just hope Sedit's news about "improved" formulations doesn't come to pass. |
Do a quick bit of research before dismissing others' claims.
http://www.imperialinc.com/msds0055120.shtml
http://www.imperialinc.com/msds0055060.shtml
Both list 1% proprietary additive on top of 99% of the respective alcohol. The bottles do advertise a rust inhibitor, which is usually some sort of
high boiling nonpolar substance.
See posts by Smuv and Mumbles: http://www.sciencemadness.org/talk/viewthread.php?tid=7990
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Do a quick bit of research before dismissing others' claims |
I did more than a "quick bit" of research. If you read my post, you see that I compared HEET to ACS solvents in several preps and obtained the same
results.
That is research, dude. I dunno why you think I hadn't looked at the MSDS. But 99% you're quibbling about?
I didn't dismiss anybody's claims. Just pointed out that what you were doing was, well, Redundant for anything except HPLC. I stand by that
assessment.
Seems like you've gotten mighty persnickety about purity lately.
Quote: Originally posted by UnintentionalChaos  | Guess I'll join the fun. I don't have any reagent stuff and do lots and lots of my own purification....
Behind the mortar and pestle are three oxidizers. One was purchased at a health food store, one a hardware store, and one a farm supply place.
|
http://www.sciencemadness.org/whisper/viewthread.php?tid=161...
[Edited on 3-10-2009 by entropy51]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by entropy51  |
I did more than a "quick bit" of research. If you read my post, you see that I compared HEET to ACS solvents in several preps and obtained the same
results.
That is research, dude. I dunno why you think I hadn't looked at the MSDS. But 99% you're quibbling about?
I didn't dismiss anybody's claims. Just pointed out that what you were doing was, well, Redundant for anything except HPLC. I stand by that
assessment.
Seems like you've gotten mighty persnickety about purity lately.
Quote: Originally posted by UnintentionalChaos  | Guess I'll join the fun. I don't have any reagent stuff and do lots and lots of my own purification....
Behind the mortar and pestle are three oxidizers. One was purchased at a health food store, one a hardware store, and one a farm supply place.
|
http://www.sciencemadness.org/whisper/viewthread.php?tid=161...
[Edited on 3-10-2009 by entropy51] |
Do you really have an issue with my desire to work with reagent-ish grade materials? I'd like to avoid any unexpected side reactions due to
impurities. Other complications might be difficulties with crystallization or depression of the product's melting point. Characterizing and purifying
the reaction products is often half the battle.
Sure, heet may work as-is for reactions, but that residue of rust preventative is going to bug me to no end and I'd kick myself if it caused problems
later on. The drying was probably redundant, but I have pounds of MgSO4 so why not? My hardware store acetone was slightly wet when I got it, so why
should I expect heet to be magically anhydrous?
In this case, the impurity is known, and a way to remove it that isn't completely unreasonable is known. Why not give my future reactions an ouce of
prevention?
And if you're hinting at it; no, nobody ingests any of my reaction products. I'm just a bit (or very) anal.
At any rate, the three oxidizers are KNO3, NaNO3, and 35% H2O2.
The H2O2 is food grade and is now stored in a fridge. The NaNO3 and KNO3 are recrystallized fertilizer and stump remover respectively.
The bench is also much cleaner and more heavily stocked than it was at that time. Back then, I didn't even have a full distillation setup. I still
don't have a fume hood, but it's on the short list.
Go find someone who's worth your time to harass.
[Edited on 10-4-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
I know this thread is a bit old, but I stumbled across a paper that describes the preparation of aluminum isopropoxide without the mercury catalyst,
instead using anhyd. AlCl3.
Richter, M. 2009. A New Catalyst for the Synthesis of Aluminum Isopropoxide. Chimicke Listy. 103 6:511-513
Unfortunately the paper is in Czech, and I can't seem to find another version of it. Also, link here http://www.chemicke-listy.cz/docs/full/2009_06_511-513.pdf
If this works, AlCl3 might boil off prior to the isopropoxide, making it difficult to completely rid the apparatus of other residues prior to the last
fraction being distilled. Still, might be worth it if you don't have to handle mercury-anything.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
why would you make something as simple as Al isopropoxide with something as hard to obtain (in sufficient quantities) as anhydrous AlCl3?
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
I wasn't aware that anhyd. AlCl3 was hard to get, and I only remembered about this thread as I was browsing through journals.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Anhydrous AlCl3 poses a problem for some people. For others it is not such an issue. It is easily made from the elements, with careful drying of the
chlorine gas and exclusion of moisture from the apparatus. Thanks for sharing the reference aonomus, despite it being in czech. Perhaps we have a
member here who is able to translate for us. The key advantage to this preparation is the lack of mercury salts as a catalyst; many people would
rather not work with them, myself included.
Picric-A: Aluminium isopropoxide is not "simple"... It is a versatile reagent in organic synthesis. Sometimes it may be difficult to appeciate its
scope. Are you aware that it can effect the attached transformation? You might like to think how it does so, and more importantly, why...
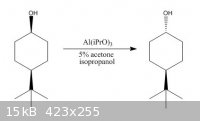
[Edited on 17-11-2009 by DJF90]
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Sorry i meant simple as in simple to make, dry isoproypl alcohol, add clean aluminium turnings (cleaned in HgCl2 (aq) ) then reflux till dissolved,
distill off isopropyl alcohol then aluminium isopropoxide - simple!
As i said AlCl3 is also 'simple' to make via combination of the elements however more tricky in making usable amounts.
Sorry for any confusion.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
While not as easy to make in bulk as the isopropoxide, the anhydrous chloride is still suitable for the preparative scale... 100g or so if you have
the apparatus large enough for it (a combustion tube and a collection chamber with drying tube attached). If you make it on a "as needed" basis, then
you can easily prepare the quantities you need (possibly even easier than the isopropoxide preparation).
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Aluminium Isopropoxide Synthesis
As stated in the first page of this thread, I need Al(OiPr)3 for some Oppenhauer oxidations.. And Panreac is out of stock, so I won't be receiving my
reagent until at least 3 weeks.. So I decided on preparing it myself.. Here is the report.
The procedure followed was that of Vogels 5th Ed., p 440, at 1/10th the scale.
All glassware used was oven dried 30min prior use.
IPA was dried by refluxing, then distilling over CaH2.

2,7g of aluminium foil was pressed as a small cigar then cut into small peices, then dried in the oven for a few minutes.
They were then placed in a 100mL 3-neck RBF, along with a stir bar, mounted with a addition funnel, a reflux condenser and a CaCl2 guard.

20mL of IPA were added to the flask via syringe.
50mg of HgCl2 were added to the addition funnel, followed by 10mL IPA. The salt was dissolved by heating with a hair dryer. The solution obtained was
then added to the stirred aluminium, and the addition funnel removed. The flask was then immersed in a 100°C oil bath.


The solution soon started bubbling and turning grey. The flask was heated to reflux, the bubbling becoming stronger and stronger. The solution became
completly turbid from the grey particules formed. The flask was refluxed until all the aluminium was dissolved ( 2H).

The flask was then left to cool overnight. The next morning, crystals had formed.

The flask was heated to dissolve the isopropylate, and transfered to a dried distillation setup.


A CaCl2 guard was placed between the aspirator and the setup.

The excess IPA was removed under weak vacuum until the mixture started getting very thick and foamming.

The residu had to be relocated in a larger flask to avoid excessive foamming, using dry IPA to rince the solids. Once all the IPA was removed, the
thick sludge melted to a easily-stirred solution. The condenser was then removed, and a clean receiver attached directly to the adapter. Vaccum was
increased, and heating continued.

At 158°C, a clear distillate started to be collected. Heating was diminished to afford a slow take off. The distillate was collected until only a
solid residu was left in the still flask.


Thus 17,3g (84.7mmol) of aluminium isopropoxide was collected, giving a yield of 84,7%, not bad!
The yield is less than stated by Vogel surely because of the several relocations into a different flask, and the fact that a rather large still flask
was used (there was alot of product refluxing when take off ceased).
The liquid did not solidify upon cooling.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Thanks for the full and graphic details, Klute. However, there is one apparent error in the accompanying text, viz. "IPA was dried by refluxing, then
distilling over CaH2"; - isopropanol would react quite violently with CaH2 to form calcium isopropoxide and H2 (and Ca(OH)2 if any water is present),
in a similar manner to the reaction with Ca metal. So you probably mean anhydrous CaCl2, or some other anhydrous Ca++ salt that readily takes up water
(this could include CaO although its heat of hydration to Ca(OH)2 is quite high), used as a dessicant to remove H2O as an hydrous salt which
preferentially dissolves in H2O rather than in isopropanol. Also, because of the approx. 70% constant-boiling-point azeotrope that H2O and isopropanol
form, you could not have gotten past this composition if you started with commercial "rubbing alcohol" by simple distillation alone.
[Edited on 15-1-10 by JohnWW]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I'm not sure John; Armarego says:
"Most of the water can be removed from
this 91% isopropanol by refluxing with CaO (200g/L) for several hours, then distilling. The distillate can be
dried further with CaH2, magnesium ribbon, BaO, CaS04, calcium, anhydrous CuSO4 or Linde type 5A
molecular sieves." 
Klute, I think you have better equipment than I have seen in any university!
[Edited on 15-1-2010 by sonogashira]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Klute is back in the lab 
Rubbing alcohol also comes in a 99% grade with a small amount of mineral oil added.
Could AlCl3 be formed in situ by bubbling chlorine into dry IPA and aluminum powder? Nah, the Cl2, HCl and a lewis acid would chlorinate everything

Perhaps the AlCl3 could be formed in ether or DCM first then IPA added after. AlBr3 might be easier to make by adding Br2. All this to replace 50mg of
Hg.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Indeed, I used CaH2, not CaCl2, it hardly bubbles at first, surely the water, then stays as is, it doesn't react with the alcohol, the still was left
2 days and the hydride reacted vigorouslyw ith water..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Any idea on what was left in the brown/black residue? Also (yes, wiki is not the best source, but its fast), why is the aluminum isopropoxide product
a liquid that doesn't solidify?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
The isopropoxide finally solidified to a gelatinous cristalline mass, it's just that it has tendency to supercool.. I prefer it liquid it's easier to
handle!
And yup, Klute's back in the lab!! 
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Maybe its highly soluble in IPA, and not all of it was removed?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
No, no, all the IPA was removed before the isopropoxide started refluxing in the distillation setup. The setup was dry when distillation started. And
it's not that soluble in IPA as it cristallized out from the solution upon cooling..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I recently conducted a large scale synthesis of aluminumn isopropoxide. A bottle of iso heet which is claimed to be 100% 2-propanol was poured into a
1L round bottomed flask. The liquid was brownish in color. 4A molecular sieves were then added and a solvent still was attached. The 2-propanol was
refluxed for 3 hours and then distilled into another flask. The distilled alcohol was dried overnight over 4A molecular sieves.
27g of aluminum foil was cut into small pieces and place into a 1L round bottomed flask. 290 mL of the redistilled 2-propanol was added, along with
0.84g of mercuric iodide. The flask was swirled to disperse the mercury salt. A long Allihn condenser was attached, bearing a CaCl2 tube. The mixture
was heated to reflux, causing the mixture to turn to a greyish color. The heat was removed and the mixture refluxed by itself. After the initial
reaction had subsided, the heating was re-applied and the mixture was refluxed for 4 hours, the aluminum had all dissolved at this point. The heating
was removed, and after standing over night, crystals of the crude product had formed. The mixture was then distilled at atmospheric pressure until all
of the remaining 2-propanol had been removed. The vacuum adaptor was then directly attached to the distillation head and the isopropoxide was then
distilled under reduced pressure using a Buchi portable water aspirator without a CaCl2 tube. The aluminum isopropoxide was collected between 160-180
degrees Celsius as a clear, viscous liquid. The product weighed 172 grams and the percentage yield based on the aluminum used was 84%.
[Edited on 25-1-2010 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
unome
Hazard to Others
  
Posts: 134
Registered: 17-10-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | Quote: Originally posted by aonomus  | I know this thread is a bit old, but I stumbled across a paper that describes the preparation of aluminum isopropoxide without the mercury catalyst,
instead using anhyd. AlCl3.
Richter, M. 2009. A New Catalyst for the Synthesis of Aluminum Isopropoxide. Chimicke Listy. 103 6:511-513
Unfortunately the paper is in Czech, and I can't seem to find another version of it. Also, link here http://www.chemicke-listy.cz/docs/full/2009_06_511-513.pdf |
I was wondering if it would be possible, given that the Hg salts are only there to remove the oxide layer, thus allowing the alcohol to come into
contact with the bare metal, if it would be technically possible to utilize a solution of NaOH/iPrOH (which would according the equilibrium be
composed also of NaO-i-Pr and H2O), which would clean the oxide layer off the aluminium, and activate it.
|
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by unome  |
I was wondering if it would be possible, given that the Hg salts are only there to remove the oxide layer, thus allowing the alcohol to come into
contact with the bare metal, if it would be technically possible to utilize a solution of NaOH/iPrOH (which would according the equilibrium be
composed also of NaO-i-Pr and H2O), which would clean the oxide layer off the aluminium, and activate it.
|
That would hinge entirely on whether sodium aluminate is soluble in isopropanol, which I suspect it isn't.
I strongly suspect that gallium salts would work here as a nontoxic alternative, however. A small amount of iodine might also work.
[Edited on 1-25-10 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by UnintentionalChaos  | Quote: Originally posted by unome  |
I was wondering if it would be possible, given that the Hg salts are only there to remove the oxide layer, thus allowing the alcohol to come into
contact with the bare metal, if it would be technically possible to utilize a solution of NaOH/iPrOH (which would according the equilibrium be
composed also of NaO-i-Pr and H2O), which would clean the oxide layer off the aluminium, and activate it.
|
That would hinge entirely on whether sodium aluminate is soluble in isopropanol, which I suspect it isn't.
I strongly suspect that gallium salts would work here as a nontoxic alternative, however. A small amount of iodine might also work.
[Edited on 1-25-10 by UnintentionalChaos] |
Adding a couple of I2 crystals works like a charm and the AlI3 product is easily seperated form the isopropoxide.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Are you speaking from personal experience or voicing a reference?
[Edited on 26-1-2010 by DJF90]
|
|
|
unome2
Harmless

Posts: 14
Registered: 21-5-2009
Member Is Offline
Mood: No Mood
|
|
Sorry to be using this acct (usually unome, but not on this laptop - finally got the sucker working again ) )
Here is a reference for using I2 or FeCl3 IIRC to make aluminium isopropoxide
[Cannot get it to upload, so 4shared will have to do ] ]
[Edited on 26-1-2010 by no1uwant2no]
|
|
|
| Pages:
1
2
3
4
5 |