| Pages:
1
2 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I thought the swern oxidation proceeded via the chlorosulfonium ion? i.e. (Me)2S(+)Cl (the positive charge is on the trivalent sulfur, hence the
chlorine is bonded to said sulfur).
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
That's correct. Sulfur is +1 throughtout the reaction until the last step, when dimethylsulfide leaves. Whatever you want to call that chlorinated
species, I'm not very knowledgeable of the strictly correct terminology.
[Edited on 22-2-2009 by Arrhenius]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ionic, huh?
New one to me. The compound I am most familiar with is Cl3C-S-Cl which is covalent. Also SCl2 and S2Cl2, the sulfur chlorides, also covalent.
But always happy to learn something new.
I will have to take a closer look at the Swern.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I don't know if I would call it ionic, judging purely from the proximity in electronegativity of oxygen, sulfur and chlorine. It's typically drawn
this way, as the "ylide", in the mechanism to emphasize the "resonance form" that is highly electrophilic at the sulfur. DMSO is drawn with charge
separation between the oxygen and sulfur, and the chlorine depicted as - and the sulfur + too. It's a pretty cool mechanism in total, and a
challenging but robust reaction.
I guess I overlooked this... but... what are you trying to run a Swern oxidation on? There are a few oxidations that are easier, and also don't
utilize chromium. Manganese dioxide comes to mind.
[Edited on 22-2-2009 by Arrhenius]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I just woke up. Of course this has to have a formal charge and of course it is an ylide.
Sic gorgeamus a los subjectatus nunc.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by Sauron
Also notice that while TCCA contains three carbonyl oxygens, TCT does not. TCT contains no oxygen.
TCT is a chlorinating agent
TCCA is primarily an oxidizing agent with some utility as a chlorinating agent as well.
They derive from the two tautomers of cyanuric acid.
TCT from cyanuric acid
TCCA from isocyanuric acid.
See structures below.
Only TCCA is a pool chemical. Put TCT in your pool and no one will ever show up to swim again.
[Edited on 22-2-2009 by Sauron] |
Just to clarify a bit 3 H atoms are missing in the structure of the isocyanuric acid, one H on each N would be better.
TCCA is related to urea,biuret and imides (-NH-CO-)3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You are right. MDL Isis Draw only adds hydrogens to heteroatoms if you change the default setting. Sorry about that.
If this were not the case, Cl could not substitute on those Ns.
Sic gorgeamus a los subjectatus nunc.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I've attached the mechanism for the Swern oxidation. I'm hoping its not too big.
[Edited on 23-2-2009 by DJF90]
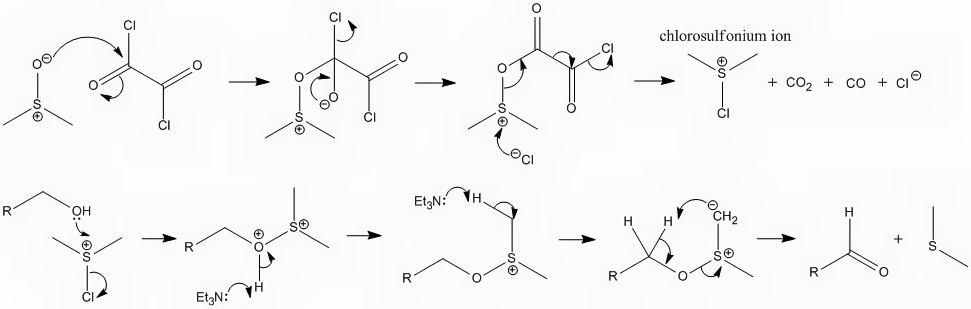
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Hmm... Yes... I wouldn't draw triethylamine abstracting a proton from the dimethylsulfonium ylide (last step), I would simply draw it abstracting the
alpha proton, to undergo an E2 type elimitation to form the carbonyl. That would be the general theory of most oxidations to the aldehyde; adding a
good "Lv" group to the alcohol so the proton can eliminate.  But I mechanisms
are just some smart guy's best guess But I mechanisms
are just some smart guy's best guess 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its a good point but I think if you consider which of the hydrogens is more acidic you will find it to be the one I have shown being removed. The (+)
on the sulphur helps to stabilise the resulting carbanion, whereas the oxygen has a positive mesomeric effect on the alpha position, DEstabilising the
carbanion that you claim would form. Like you say mechanisms are only "best guesses" but this is the one I believe to be more accurate.
|
|
|
voidwalker
Harmless

Posts: 4
Registered: 12-9-2009
Member Is Offline
Mood: No Mood
|
|
How can you make oxalyl chloride from cyanuric chlroide and oxalic acid ? I've searched the internet for refferences but can't find anything.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
To go back to the argument about activating reagents, you can use a whole range of activators for the Swern oxidation, including oxalyl chloride,
thionyl chloride, trichlorotriazine (TCT), acetic anhydride, Pyridine-SO3 complex, benzoyl chloride, tosyl chloride, triflic anhydride, PCl3 and on
and on... I'm fairly sure (though not 100% certain) that TCCA (pool chlorinator) is no good in this reaction, however.
In any case, as a general rule, oxalyl chloride gives the best yields under reasonable conditions (i.e. not requiring co-solvents like HMPA!) over the
other reagents - hence its widespread use in the reaction (in fact, I can't remember the last time I saw a Swern using other than oxalyl chloride!).
The trick to good yields with the Swern is to control the temperature fairly well - sit by your bath and maintain it at -60*C. Since the reaction
usually takes less than an hour, it's not a real hardship.
Be aware of the stench on work up, too - the formed dimethylsulfide is rather unpleasant to work with! Best done in a hood, outside if necessary
(although the neighbours may not approve!). You're also going to want to remove the solvent from your product in a hood or outside.
Also, dump all your glassware in a bucket full of bleach for a while before cleaning them, and add a bit of bleach to the aqueous extracts from the
work up - bleach will oxidise the DMS to non-smelly compounds.
For a comparison of the various activating reagents, see Omura and Swern, Tetrahedron 34 (1978), 1651-60
Attachment: Comparison of reagents for use in Swern oxidation.pdf (928kB)
This file has been downloaded 1444 times
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
For a start, you will only EVER see oxalyl chloride as the activator in a Swern oxidation; this is because by definition the reagent couple in the
Swern oxidation is (COCl)2 and DMSO. Albright-Goldman oxidation uses Ac2O/DMSO, while Parikh-Doering uses the SO3-Py/DMSO. Pfitzner moffat uses
DCC/DMSO/Pyridinium trifluoroacetate (or in some cases H3PO4 to advantage). Corey Kim oxidation originially used Cl2/Me2S, but a modern modification
uses Me2S and NCS. I could go on for a while as there's quite a few oxidations relying on "activated DMSO"; Swern oxidation is just ONE of them.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
DJF is right, of course... I should have said a Swern-type oxidation using the other activators, with a few exceptions (the Parikh-Doering, in
particular, has become somewhat more popular lately). I often forget the fact that the reactions have different names - ultimately, the mechanism is
more or less the same, so mentally I lump them all together! But there are definitely differences, and they can be important depending on the material
you're working with. Apologies for the slack terminology!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
It's fine, you're right, they all have roughly the same mechanism; DMSO + Activator => Activated DMSO, Activated DMSO + Alcohol =base=> Aldehyde
+ Me2S. The exception of course is the Corey Kim, which starts from dimethylsulfide, and, in the traditional method (using Cl2), the activated species
was the same as that in the swern oxidation (chlorodimethylsulfonium chloride). You are also correct when you say that the Parikh-Doering methodology
has become fairly popular lately - SO3-Py is much more pleasant to handle than one would expect given it's constituents, and the reaction is actually
rather clean (no chlorination as can occur with Swern conditions). Of course, the main drawback with activated DMSO type oxidations is the generation
of Me2S, which must be absorbed by a scrubber.
[Edited on 8-4-2010 by DJF90]
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
I bought (COCl)2 from Sigma withouth any problem!
|
|
|
| Pages:
1
2 |