| Pages:
1
2 |
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I would strongly recommend to use CCl4 not DCM in spectroph. measurements. CCl4 cannot be radically H abstracted and serve as a part of radical
reaction (decomposition) chain.
I have just found interesting paper (ACS, ja00381a032). Mixture Cl2O in CCl4 + CF3COOH or H2SO4 is using for chlorinatinon of toluenes to
ring-substituted toluenes. Without acid, chlorination takes place only at α-C of aliphatic chain attached to benzene ring.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I would agree. Although I was planning on doing the UV-VIS on the concentrated acid:-OCl mixtures rather than the DCM extracts (for that reason). I
have observed DCM couple to yield tetracholorethylene (and ethanes) when the yellow extracts are subject to light.
So, it looks like the species is nice and electrophilic and substitutes from the ring, rather than at the a-position (which is a hallmark of a radical
species). Did they mention if light was present, or simply -OCl and strong acid?
What I might do, however, is to scan the irradiation wavelength vs. fluorescence to *hopefully* find the wavelength that cooks it.
I have been so slammed in the lab since the holidays. Everything died/has gone wrong and work is backed up. I'll get to the instrumental while I can.
The reaction seems to go just fine regardless of the acid used, eg. H2SO4 or HCl wrt to the yellow, reactive extracts.
Cheers,
O3
[Edited on 6-1-2009 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by Jor
I assume you mean Ca(ClO)2 ? |
Yes, Ca(OCl)2, I edited the former post.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ozone
Did they mention if light was present, or simply -OCl and strong acid?
|
It is hard to say if ligh was present.
The paper in attachment + spectral data of Cl2O (from earlier mentioned K-O encyclopedia).
Attachment: aaa.pdf (155kB)
This file has been downloaded 963 times
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Interesting paper, KMnO4.
The footnote #5 indicates that "...free radical initiator or UV light had little or no effect..."
This is inconsistent with Woelen's findings which are consistent with mine. I do not think that Cl2O is involved, and the following experiments help
to secure this position.
Reagents:
Chlorox Ultra, "6% NaOCl". I did not have time to standardize it via H2O2.
HCl, Mallinkrodt AR Select Trace Metal grade, 36.5-38%.
Water, Nanopure, 18.3 MOhm filtered through a 0.2um membrane. All solutions are aqueous and made using this water.
A solution of bleach was made to contain 1.2 % (DF 5) "NaOCl". Because the sealed cells were no where to be found, and I am not putting fuming acid
into the spec unsealed, I made a 1:1 solution of HCl.
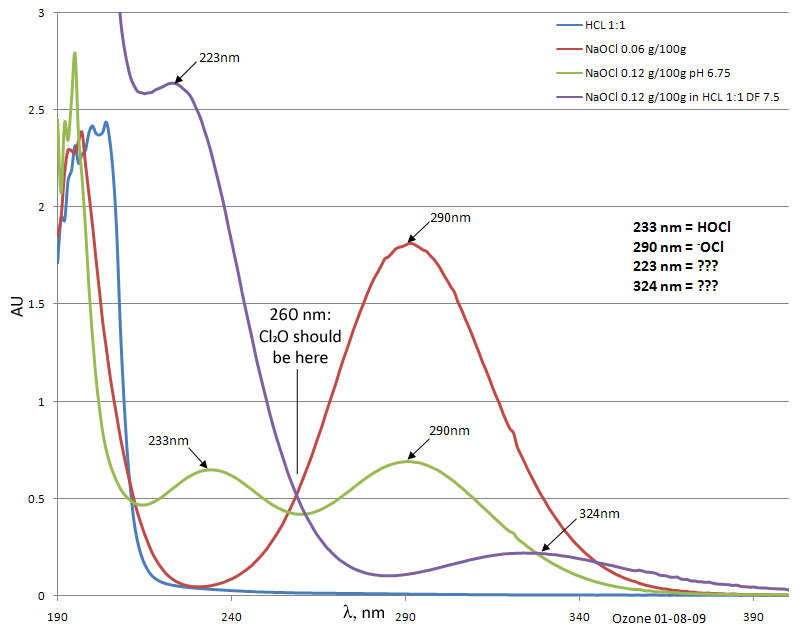
1. The absorbance of 1mL 1:1 HCl was measured from 190-500nm at 1200 nm/m using a Beckman-Coulter DU-800 spectrophotometer. The resulting spectrum was
featureless until reaching the UV-cutoff of the 6Q cell.
2. The 1.2 % NaOCl solution exceeded the linear range of the detector by a factor of ~twenty. A scan of a dilution to 0.06% gave the expected peak at
290 nm.
3. 0.12% NaOCl adjusted from pH 11.00 to 6.75 was scanned. Note the evolution of the HOCl peak at 230 at the expense of the -OCl peak at 290.
4. To 900 uL of 1:1 HCl was added 100 uL of 1.2% NaOCl. Neither HOCl nor -OCl were detected. Instead new peaks at 223 and 324 evolved. These exceeded
the detector range and so were run again at DF 10. The plot indicates a curve of this result multiplied by 2.5 for scale. Addition of EtOH caused the
size of these peaks to decrease in size. The expected Cl2O peak (Marsh, et al, 1982) at 260 nm was not evident.
Conclusion:
HOCl is NOT the oxidizing species resulting from the treatment of NaOCl (which Woelen established is equivalent to Ca(OCl)2 in terms of activity) with
1:1 HCl.
The species is oxidative and is probably labile to light. 223 and 324nm are good wavelengths to use in further experiments (a mineral light with 254
and 365 will probably do).
The oxidative species is not Cl2O. The two species evolved are different from HOCl, -OCl or Cl2O. I need to scan chlorine water (unless someone has
the spectrum handy).
This was a two-beer post.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Chlorox can contain nearly as much chlorate as hypochlorite, a little chlorite, and of course lots of chloride.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks,
Yes, I am aware of this. It was still better than my pool shock with 45% "inert ingredients".
I have some 12% Na hypochlorite from Aldrich. I had previously tested this vs. Chlorox wrt. UV-VIS. The results were identical. I'll standardize both
the bleach and the Aldrich material against H2O2 (standardized vs. thiosulfate *sighs*) when I get the chance.
The test sequence provided above is not too intensive and can be repeated with any number of refinements.
It would be a good idea, though, to also scan some NaClO3 for reference. I'd think that the chloride in the presence of strong HCl would be moot?
Chlorite was not seen in the spectra.
I am interested in determining some stoichiometry and reactivity of the material with and without light and/or various oxidizable substrates. Of
particular interest, it appears, would be toluene which we can use to differentiate between aromatic or aliphatic chlorination.
I wish I had some ethyl or propyl benzene so I could be more sure (benzyl groups tend to have their own specificity).
I do have styrene, though . .
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Ozone, what you could do is take a dilute solution of NaOH (keep it cold!) and slowly bubble Cl2 through this. In this way you make a fresh solution
of NaOCl and the chloride and hydroxide which will be in this solution is not a problem at all. When the solution is kept cold while Cl2 is bubbled
through, then you won't suffer from formation of NaClO3.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Ozone, you lead me into depression with these maesurements.
But not beacuse of same spectra but beause of possibility of their creation. I would like it too... 
I attach another literature which brings a little (green) light on your spectra.
Page no.2 is from K-O encyclopedia and reference (13) is on the first page.
Now discussion.
Cl2O exists in H2O solutions mainly as HOCl. .
But there is (fast) equilibrium Cl2O <-> HOCl and K=[Cl2O(aq)]/[HOCl]<sup>2</sup> is from 1/1000 to 1/100
(data from review cited earlier). From K-O encyclopedia:
Carbon tetrachloride (CCl4) extracts chlorine monoxide but not HOCl from concentrated HOCl solutions. The partition coefficient at 0 C for the
equilibrium Cl2O(aq)<->Cl2O(CCl4) is 2.22.
2.22 is rather a small value 
If you detected HOCl, then equilibrium amount Cl2O were also present, but below level of detection under these conditions.
To prove anything, measurements must be conducted in CCl4 (or something similar) extracts, not in water.
BTW footnote no.5 says about Cl2 ( a note for those who did not read paper)
[Edited on 9-1-2009 by kmno4]
Attachment: data.pdf (80kB)
This file has been downloaded 604 times
|
|
|
| Pages:
1
2 |