| Pages:
1
2 |
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
CHLORATES HAS NO PLACE IN AMATEUR ROCKETRY. PERIOD.
This cannot be stressed enough. Chlorate compositions can be extremely sensitive to heat, impact and friction. It's one of the fastest routes to
explosions, failure and injury.
@B(a)P: The reason why chlorate and wax isn't more spectacular is due to the low boiling point of the wax. It acts like a cooling bath, preventing the
positive thermal feedback that other mixes are subjected to. But don't let that fool you, it's still a high explosive mixture.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
@Fulmen - Thanks for that. Wax has quite a high enthalpy of fusion so presumably that is a factor also.
Rocketry is of interest to me from a theoretical perspective, I can't put it into practice as I live in the heart of a large city.
I have read extensively on it and have never come across a propellant that includes chlorate and pretty much every text I have read indicates, as you
say, that chlorates are a terrible idea, so I was curious to understand where @Nitrosio had found this information.
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fulmen  | CHLORATES HAS NO PLACE IN AMATEUR ROCKETRY. PERIOD.
This cannot be stressed enough. Chlorate compositions can be extremely sensitive to heat, impact and friction. It's one of the fastest routes to
explosions, failure and injury.
@B(a)P: The reason why chlorate and wax isn't more spectacular is due to the low boiling point of the wax. It acts like a cooling bath, preventing the
positive thermal feedback that other mixes are subjected to. But don't let that fool you, it's still a high explosive mixture.
|
Thank you!!
This needed to be said. Chlorate mixtures can be insanely sensitive, even when plasticized.... as in primary explosive sensitivity. That’s what test
confirm. Even when in putty form. Just. Don’t. Stop
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fulmen  | CHLORATES HAS NO PLACE IN AMATEUR ROCKETRY. PERIOD.
This cannot be stressed enough. Chlorate compositions can be extremely sensitive to heat, impact and friction. It's one of the fastest routes to
explosions, failure and injury.
@B(a)P: The reason why chlorate and wax isn't more spectacular is due to the low boiling point of the wax. It acts like a cooling bath, preventing the
positive thermal feedback that other mixes are subjected to. But don't let that fool you, it's still a high explosive mixture.
|
Thank you!!
This needed to be said. Chlorate mixtures can be insanely sensitive, even when plasticized.... as in primary explosive sensitivity. That’s what test
confirm. Even when in putty form. Just. Don’t. Stop
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Amateurs like stuff that is easy to get or make. Hence chlorates and all substitute stuff is so much sought upon. It'd be wise to say that either do
it good or don't do it at all.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
@Fyndium....Holy words.
KClO3 + asphalt is only error in the digit. Fake news, which is repeatedly interpretation from old spread source.
Mixture exist, but is it KClO4 + the asphalt. Beginers catching this desinformation and use as rescue rope because they have KClO3, which is easy
available. KClO3 + the asphalt is desinformation a like rocket fuel.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I filled a small glass 10 ml vial with KClO3 and sugar mix expecting a small boom and instead I got a rocket.
I think a rocket could be made, might need to taper the mix to be less volatile from the start point to the end point to prevent it from accelerating
into a boom.
Maybe forget about a core burner , thinking that for end burner add more sugar towards he back of the thing to slow it down.
No one says you have to use the same mix for the whole thing.
[Edited on 8-2-2021 by Pyro_cat]
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Pyro_cat: NO! Could you please stop talking and start listening?
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I think I am ready.
Made some flash yesterday out of the chlorate I made from bleach + al and garden sulfur.
Small pile on the test plate like I have done before very bright but not very impressive slow burn. The hammer tests same as usual.
Lets see what happens if I put a few grams in that little glass vile no real top just a loose tin foil plug.
My prediction was with the course particle size maybe a hiss of gas escaping followed by small boom of the container failing.
Glad I am safety minded and had it behind that big rock with long fuse, that was old school m80 level energy release, damn !
Chlorate is nasty. I get it now.
[Edited on 9-2-2021 by Pyro_cat]
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
If there is a next time no sulfur. I know its not recommended. It should work without it.
I will find out, experiments more fun then asking the forum all the answers.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
True, experiments are usually more fun than asking questions/reading about theory. Unless they're like J_sum1's experiment where he had less than a
gram of an experimental chlorate mixture ignite during mixing and blow a hole in the palm of his hand. 
Somewhere theres a (possibly incomplete?) list of things that will cause high sensitivity or even worse, variable sensitivity or even delayed
ignition when mixed with chlorates. I want to say aluminum was one, but perhaps not as fickle as sulfur that gradually builds up acids on its surface.
As far as rocket propellants go, is there any clear advantage against KNO3? If you have to use a less-than-ideal ratio of KClO3 with a second-rate
fuel for safety reasons, you're probably back to a weak propellant. Maybe better to play with nozzle and grain geometry? I haven't done much in the
way of pyrotechnics, and I'm no rocket scientist so take that with a grain of saltpeter.
[Edited on 9-2-2021 by Vomaturge]
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
Fully shielded I put a drop of sulfuric acid on tiny pile of chlorate and sugar mix and it quickly ignited. I am guessing that besides the sensitivity
issues that reaction is why you keep sulfur away from chlorates.
Keep meaning to try making successful sugar KNO3 rockets. Made the mix and experimented but never made a successful rocket. Only 2 low effort tests
before I started on the thermite project.
This should be my next project.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
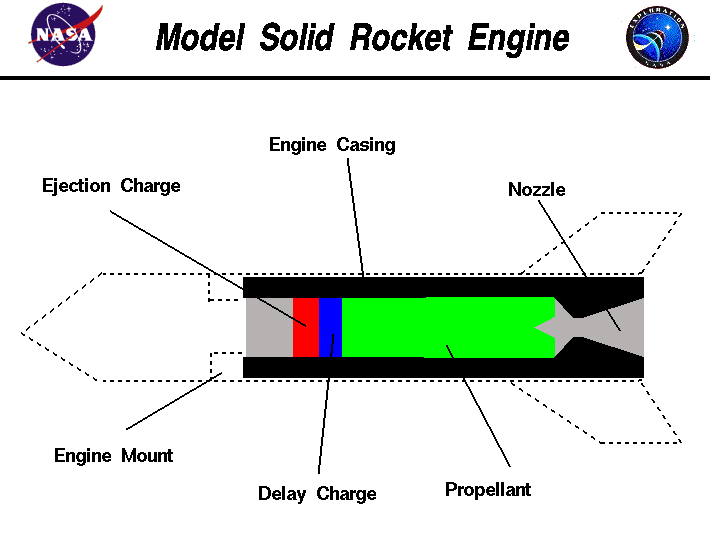
A quality rocket engine was developed in the Laboratory of Liptakov. Basic data: Engine housing 122g, engine capacity 150g classic BP. With a density
of 1.85 - 1.95 g / cc. Thrust time 10 seconds. Thrust average 1.2 Kg. Start peak thrust 2,1 Kg / 0,5 sec. Total impulse 120 Ns. Construction - end
burner type. Used here: https://www.youtube.com/watch?v=Z17iQNabhbk
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
NH4ClO4 + Al is explosive. NH4ClO4 + Al + Binder is ok.
KClO3 + a good Binder for Propellant is possible, not?
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 175
Registered: 12-7-2018
Member Is Offline
|
|
I ve heard where i live they used Newspaper soaked in concentrated Chlorate solution as an rocked fuel. I think Rolled toiletpaper or newspaper can
also used as gun propelant if you hold it on max. concentration and same temperature. Other problem with chlorates is if the inner presure rises to
much it detonates. Students made roman candels with the stuff first time it shots it the flares and than boom.
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
AN + Paper is for Rockets. KClO3 + Paper ist for Canones and Salutes etc.
|
|
|
Industrial Adhesive
Harmless

Posts: 15
Registered: 30-11-2018
Location: to be honest, I lost track
Member Is Offline
|
|
check out a guy named Richard Nakka https://www.nakka-rocketry.net/ he has been doing rocketry for a very long time and has all the info that you need. also check out some amature
rocketry fourms. there are pleanty of other cool rocket propellants that are used commonly used such as nitrocellulose colloid which is either left as
is or mixed with another additives https://patents.google.com/patent/US3617400A/en https://history.nasa.gov/conghand/propelnt.htm
also have you ever thought of attempting to make a hybrid motor? they are pretty cool. and if you cant get a hold of common oxidizers thats a good
option.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I still think the chlorate rocket is feasible.
Every rocket even NASA, everyone gets far away from the thing before launch in case it blows up.
very small scale but I put some of my Chlorate mix in a plastic wire nut and it behaved like a rocket. It seemed like it went for the plastic instead
of the aluminum cause it was easier to get at as fuel.
I was not even thinking it at the time but the plastic in a wire nut I would imagine is designed to resist combustion that might be useful in slowing
it down,
Its behavior reminded me of the Oxygen in the acrylic tube video.

|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Even if aluminum fronts and steel bolts are used, the engine will be heavy. In amateur practice, engines are successful where the engine body (+
necessary parts) is lighter than fuel. Every gram of solid engine components determines engine efficiency. Another issue, is the specific impulse of a
given fuel.
If you look at the Nakka website, you will not find chlorates there.
[Edited on 11-4-2021 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
The simplest way to prepare KClO4 is to melt KClO3. if it doesn't go off, it will turn into a mix of KCl and KClO4.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I don't reccomend chlorate for propellant uses, all the above comments about tendency towards CATOs, blown up gun barrels & other accidents are
typical.
However, there has been some use in the past, I particularly recall the Selkuk Oztap article on whistles, including drivers and rockets from
Pyrotechnica IX:
https://www.google.com/url?sa=t&source=web&rct=j&...
Attachment: Whistles_Pyrotechnica_Xi.pdf (4.1MB)
This file has been downloaded 455 times
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Fair enough, but that's only half the problem. First you need to make the propellant, and chlorate compositions tend to be sensitive. So you might
want to set up some sort of remote handling which can be much more work than making the rocket.
And even if you can have it blow up without risk of injury your rocket, the payload and possibly the launch pad is gone. That's a lot of work for a
loud booom.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I really need to experiment with KNO3 sugar rockets before I would even attempt conquering chlorate.
Mix with fire resistant plastics ? en.wikipedia.org/wiki/Fire-safe_polymers
Or the other idea is to have a pulsing rocket like a strobe rocket with slow it down with delay charges layered into an end burner similar to the slow
down stage at the end before parachute charge,
These delays might be able to stop the burn from accelerating to the point of blowing up the rocket.
I am just talking theory, I don't see myself ever trying.
|
|
|
| Pages:
1
2 |