| Pages:
1
2 |
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Blogfast25, even MINUTE quantities of manganese in higher oxidation state give a deep green/brown color. The almost black liquid in the test tube on
my webpage was made from 2 ml of 30% HCl and just 3 little specks of solid KMnO4, eack speck having a size of just 2 mm diameter, or even less. If
your MnCO3 only contains a few thenths of percent of manganese in higher than +2 oxidation state, then you'll get a dark green/brown liquid when you
dissolve this. On heating this color will fade though. The presence of iron will make the liquid appear yellowish, due to formation of the FeCl4(-)
complex, which has an intense yellow color.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Woelen:
I accept what you say but "2 ml of 30% HCl and just 3 little specks of solid KMnO4, eack speck having a size of just 2 mm diameter" is
not that dilute a solution. If Mn(III) is responsible at very low concentrations, wouldn't that in itself point to some strongly coloured
complex of Mn<sup>3+</sup>? Surely the Mn<sup>3+</sup> ion, even with water ligands cannot be that strongly coloured?
[Edited on 11-8-2008 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I do not say that no Mn(3+) complex is formed in the experiments (both yours and mine). I am saying that no such Mn(2+) complexes are formed.
A nice test would be to add a tiny pinch of Na2SO3 to your green solutions. I expect them to either turn pale pink, or yellowish (the latter if iron
is present in your solutions).
The Mn(3+) ion does not have such a strong color. I made a manganese(III) compound in another experiment in a solution, which does not contain
chloride, but sulfate only at very low pH. This ion is reddish/brown. Here follows a picture of a solution of Mn(3+) in the presence of lots of
sulfate, but no chloride:
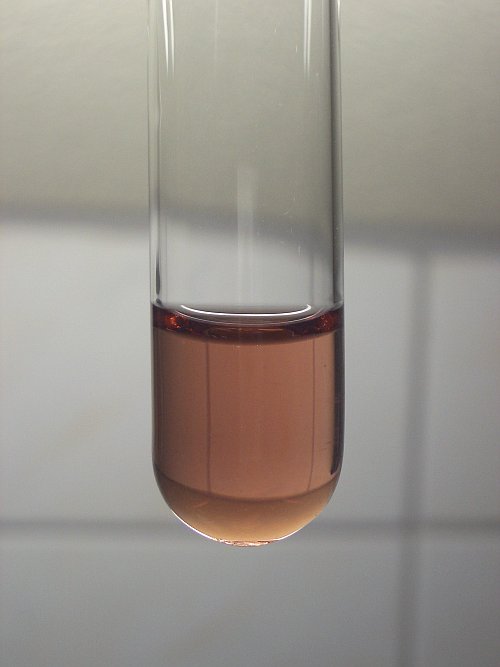
EDIT by woelen: Changed link, so that it works again.
[Edited on 12-6-12 by woelen]
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Interesting
Well, I would say that many transition metals form intensely colored chloride complexes and manganese is no exception. I guess this is the reason why
transition metal nitrates and sulfates are usualy preferred over chlorides for precipitating hydroxides, carbonates, oxalates etc. But some metals
like chromium form complexes even with sulfate where only a portion of the sulfate precipitates with addition of barium chloride  . .
[Edited on 8/12/2008 by chloric1]
Fellow molecular manipulator
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Indeed, most transition metals form complexes with chloride, but at lower oxidation states, the formation of complexes is less pronounced. Manganese
in oxidation state +2 is not at all strong in formation of complexes, but manganese in oxidation state +3 is much more so.
Chromium is a completely different story. The metal in oxidation state +3 has the special property that it not only forms complexes, but these
complexes also are very stable, and once they are formed, the ligands are not easily replaced. For instance, I have chromium(III)sulfate and also
potassium chromium(III) sulfate (chrome alum). Both are dark purple solids and solutions are purple with a blue/grey hue. When barium salt is added to
such a solution, all sulfate is precipitated. However, when such a solution is boiled for a while, then the solution turns green. The sulfate ions
then replace some of the water ligands and in that case, barium salts only precipitate part of the sulfate. It takes weeks before such a green
solution has reverted to the purple/blue solution after cooling down. A similar thing happens when dichromate ion is reduced by sulphur dioxide in
acidic medium. The sulphur dioxide is transformed into sulfate, and this sulfate immediately is coordinated to the chromium(III), formed from the
dichromate. Hence, the solution becomes green. When dichromate is reduced by e.g. nitrous acid in excess acidic medium, then a purple/blue solution is
obtained.
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I think only chromic nitrate and perchlorate are the only common non-complexing salts. Woelin, am I correct that coordinated transistion metal
compounds are covalent by nature? Or is the ionic/covalent character on a sliding scale based on valence and ligand properties?
Fellow molecular manipulator
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@Woelen
How can your be sure that what you have in your picture is Mn+++? The Mn+++ ion is a powerful oxidizer, about the same as MnO4- in acid solution, pH~0
(SEP~1.5 for both)... It is unstable at higher pH, IIRC.
Have you tried dissolving MnO2 in conc. HCl at 0C as I mentioned above? It dissolves easily to produce a very deep brownish red solution which is said
to be MnCl3 (or possibly MnCl4 even); raising the temp causes chlorine to be emitted and there is a transient stage at which a green coloration can be
seen. I have read somewhere that the ‘MnCl4’ can be extracted with ether to give a green solution. (You can’t keep ether here; it virtually
boils unless refrigerated.)
Mn+++ is far less stable than Fe+++. Like Fe(III) it is said to produce a (red) alum with ammonium, also unstable, more so the violet Fe(III) version.
As I mentioned above, MnO2 heated with conc. (98%) H2SO4 produces a dark green solution (plus O2). This is said to be Mn2(SO4)3. So what color is the
Mn(III) ion?
Regards,
Der Alte
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
According to literature, Mn(3+) can be made by careful reduction of permanganate with malonic acid. It also can be made by careful oxidation of
manganese(II) ions by any oxidizer and in the absence of chloride ions, and in the presence of malonic acid. I'm not sure why the malonic acid is
needed, but apparently it only reduces to the +3 state quickly and the final step is going much more slowly.
I have also done oscillating reactions with malonic acid and manganese(II)/(III) systems and then the solution oscillated between colorless (+2
oxidation state) and the color shown above (+3 oxidation state). All these things make me quite sure that this is the color of manganese(III) in
aqueous solution, without the presence of chloride.
When some of the above reddish solution is added to concentrated hydrochloric acid, then a green/brown solution is obtained, as shown in my webpage
from a few posts before.
If you have malonic acid yourself, please try what I have described above. It is very interesting...
@chloric: I indeed am inclined to say that coordinate bonds have a covalent nature. The ligand shares a free electron pair with the metal core. But
there also is kind of sliding scale. Some coordinate bonds are weak (labile), the electron pair is only slightly shared with the metal core, while
others are really strong and then one cannot distinguish anymore between a normal bond and a coordinate bond.
A nice example is the ammonium ion. This is formed by means of a coordinate bond between the free electron pair of NH3 and the H(+) ion. So, it is
formed thus:
H3N: + H(+) --> [H3N:H](+)
Once the bond is made, one cannot at all distinguish between the newly added H-atom and the other three, all bonds are equally strong and one cannot
tell anymore which H-atom is from the H(+). If lateron a strong base is added, and NH3 is split off again, then there only is a 25% chance that the
original H-atom from the H(+) ion is not in that NH3-molecule.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I see the 'manganese wars' are still raging - 
A little sick of working with various (and variable) sources of Mn (pottery MnCO3, battery MnO2) I'm now purchasing 250 g of
MnSO<sub>4</sub>. H<sub>2</sub>O (reagent grade, only about 5 bucks) as a relatively pure source of Mn, for conversion to
anhydrous MnCl<sub>2</sub> (via carbonate). This should be Fe-free and I'll test the green solution obtained the way as Woelen suggests
(although with metabisulphite).
And tomorrow I'll test the following dehydration procedure: dissolve (more or less) equimolar amounts of MnCl2 and NH4Cl, then crystallise and grind
up. Then fume off the NH4Cl directly at about 400 C in a stream of dry CO<sub>2</sub>.
The various batches of anhydrous MnCl<sub>2</sub> seem to differ a little in deliquescence, some remain quite dry even in open air, others
seem to 'go liquid' quite quickly. I suspect the level of Fe contamination may be causing this: I imagine anhydrous FeCl<sub>3</sub> to be
highly deliquescent.
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
That should work nicely blogsfast. Hopefully, at least some of the carbon dioxide will combine with the ammonia to leave the HCl free. If not the CO2
will at least keep the pH lower so the manganese chloride won't hydrolsize.
Fellow molecular manipulator
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks, Chloric, fingers crossed (FYI I ran some dichromate boosted thermites - see the relevant thread).
While making the first 90 g batch of this MnCl2 hydrate/NH4Cl co-crystallised mixture, I ran a couple of tests that seem to confirm Woelen's ideas.
I dissolved two 'pinches' of pottery MnCO<sub>3</sub> in separate test tubes in excess HCl and boiled both up. Before boiling both are a
reddish/brown but on heating they turn a kind of kaki green, which on cooling disappears to a light orangy-ish. On heating, the green reappears.
Adding some metabisulphite (Na<sub>2</sub>S<sub>2</sub>O<sub>5</sub> to one and the colour disappears altogether. Adding NaOH to the (hot) second one and whitish-pink Mn(OH)2
precipitated. to one and the colour disappears altogether. Adding NaOH to the (hot) second one and whitish-pink Mn(OH)2
precipitated.
The fact that the colour disappears on adding a reduction agent does indeed strongly suggest the colour is due to a labile higher oxidation state than
+II. But which one?
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@blogfast25 & Chloric: Nice to see, in the thermite thread, some interesting work being done way above the ke3wl level! Makes me want to try some
myself!
And, WRT this ion color thing we seem to have stumbled on, I found the following, which may help explain diverse things we & Woelen have discused:
| Quote: | • Mn3+ causes red and green colors in octahedral sites. Muscovite mica from Brazil containing is red as is Mn3+ in beryl from Utah, synthetic
orthopyroxene, and piemontite from Whitewater, California. Andalusite containing Mn3+ is green. In the amphibole, tremolite, from New York, it
produces a violet color.
• Mn2+ usually results in a pink color in octahedral sites. Rhodonite from Minas Gerais, Brazil, is a pyroxenoid containing Mn2+and has the typical
pink color of Mn2+ minerals. Rhodocrosite from Colorado has a high concentration of Mn2+ and a bright red color. At lower concentrations, Mn2+ causes
pale pink color. When the Mn2+ is in a tetrahedral site, then yellow-green color results such as is the case with willemite.
• Fe2+ in forsterite from San Carlos, Arizona, and in phosphophyllite from Bolivia is the ion responsible for the green color. In some minerals with
high concentrations of Fe2+, such as fayalite or orthopyroxene, the color is brown.
• Fe2+ in the square planar site of gillespite or eudialyte produces a rasberry red color.
• Fe2+ in the eight-coordinated site of pyrope garnet from Tanzania produces the near-red color.
• Fe3+ in octahedral sites causes only pale color when the Fe3+ ions are isolated from each other by intervening silicate ions, etc. Pale purple
color is found in phosphates such as strengite and sulfates such as coquimbite. Yellow-green can be found in ferric silicates such as andradite garnet
from Italy.
• Fe3+ is in the tetrahedral site of plagioclase feldspar from Lake County, Oregon, produces a pale yellow color. In an unusual variety of diopside
containing Fe3+ in a tetrahedral site, it produces bright orange color in thin section.
• Co2+ in synthetic olivine and cobaltian calcite from the Kakanda Mine, Zaire, causes a typical reddish color. In tetrahedral sites, Co2+ causes
blue color such is found in some spinels.
• Ni2+ in synthetic olivine has the green color typical of Ni2+ in an octahedral site. If all the nickel is forced in to the larger M2 site by
appropriate chemical substitution (in this case in a LiScSiO4 olivine), the color is yellow, typical of Ni2+ in large, distorted sites.
|
Source:- http://minerals.caltech.edu/COLOR_Causes/Metal_Ion/
I am surprised that Mn(III) occurs in minerals, except that it is very stable with oxygen. Not being a mineralogist(? sp.) , I have no idea of the
quoted mineral compositions. I added the stuff for other transition metal for general interest. There's a lot more on that site for those interested.
Regards
Der Alte
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by DerAlte
@blogfast25 & Chloric: Nice to see, in the thermite thread, some interesting work being done way above the ke3wl level! Makes me want to try some
myself!
Regards
Der Alte |
What's the "ke3wl level"??
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by blogfast25
| Quote: | Originally posted by DerAlte
@blogfast25 & Chloric: Nice to see, in the thermite thread, some interesting work being done way above the ke3wl level! Makes me want to try some
myself!
Regards
Der Alte |
What's the "ke3wl level"?? |
Blowing things up and making a general nuisance of yourself 
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
DerAlte beat me to the punch. Mn(II) in a tetrahedral environment is both a different colour, yellow-green, and much more intense, by several orders
of magnitude, than octahedral Mn(II). However all the references I've got state that the tetrahedral complexes are not stable in donar solvents (H2O,
ROH, RCO2H).
Mn(III) is reasonable stable in non-aqueous environments and where it is not in solution; Mn(OH)2 is oxidised by air to MnO(OH) and other Mn(III)
hydrated oxides, the white Mn(II) turning to brown. Alkaline conditions favour this, acid inhibits it.
Colour changes on heating need not indicate changes in oxidation state, many complexes change colour when heated and revert when cooled.
Given that so many posters have said that their pottery grade MnO2 contains iron, I think that the possibility that some of the colour effects may be
coming from Fe contamination, especially given the weakness of Mn(II) absorption bands.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by not_important
Colour changes on heating need not indicate changes in oxidation state, many complexes change colour when heated and revert when cooled.
|
Granted, but how to explain the colour change on addition of a reducing agent (here basically SO<sub>2</sub> ? ? 
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have the opinion that the only explanation is that Mn(III) or higher oxidation state is reduced to Mn(II). This also perfectly matches the remarks
made above, that the green Mn(II) complexes are not stable in water.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Woelen:
Hmmm... doesn't appear that clear cut to me...
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@blogfast25
Re: What's the "ke3wl level"??
I think ScienceSquirrel described it well. It was meant to be a compliment to both you and Chloric for conducting scientific investigation into high
energy reactions.
Incidentally, since I am at least 2 generations removed from them, I misspelled "Kewl", not being au fait withe their prole talk. The correct kewlese
is, I believe, k3wl. I make no apologies to them, of course.
WRT Woelen's last comment, that Laval(?) slide I gave earlier suggests that any of the complexes of Mn(II) have a low K (equilibrium const.) of
formation but can be 'extracted' by EDTA. The 'jaune-vert' (yellow-green) MnCl4 - - one might be stable in excess HCl.
But I think we are chasing a red herring here. Mn can produce about every color under the sun, and many of the transition metals do likewise.
Regards,
Der Alte
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks. Der Alte...
|
|
|
| Pages:
1
2 |