| Pages:
1
2
3
4
5 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
So it appears the eutectic is 0.67 moles of FeCl3 to 0.33 moles of CaCl2, or on a weight basis, about 3 grams of ferric chloride for one gram calcium
chloride
This is apparently a surprisingly simple ionic liquid.
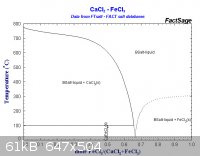
[Edited on 26-4-2012 by AndersHoveland]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Giver of bad advice, indeed  : :
Mole fraction FeCl3 = 0.67,
Thus 0.67 * 162.2 / (0.67 * 162.2 + 0.33 * 111 ) = 0.748 or multiply by 100 % = 74.8 weight %.
With 162.2 g/mol the molecular mass of FeCl3 and 111 g/mol the molecular mass of CaCl2.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I don't understand, aren't you just repeating what Anders said? Three grams out of four total represent iron chloride, ie 75% FeCl3 by
mass?
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
It is likely that Anders changed his comment when he was corrected by blogfast25.
[Edited on 4-5-2012 by barley81]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
That would not have been possible. The post from me is marked
[Edited on 26-4-2012 by AndersHoveland], while the subsequent post from blogfast25 is marked "posted on 28-4-2012". It would not have been possible
for me to alter the date of my post after I had altered it. Whenever a member alters their original post, the forum automatically adds an [Edited by
... on ... ] date stamp to the bottom of the post. These can be deleted by altering the post again, but this just adds another new date stamp to the
altered post. Even if I had deleted the whole post and posted a new post, then faked the [Edited by ... on ... ] stamp at the bottom, the "originally
posted on" date would have been later than the next post by blogfast25, which was posted on 28-4-2012. The top of my post reads: "posted on 26-4-2012
at 21:06", while the top of blogfast25's post reads "posted on 28-4-2012 at 10:10". It is not possible for a member to alter these dates. It is also
not possible on this forum to modify a post after 24 hours of time has elapsed.
In any case, I did not correct my post in response to any outside criticism, not that it matters.
[Edited on 4-5-2012 by AndersHoveland]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
I hereby find the defendant not guilty.
Dann2
[Edited by Dann2 on 1 May] 
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Sorry Anders. I apologize for misconduct.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Then there's only one possible explanation...
Time travel   JK JK
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
As part of my long-running experiment to obtain neodymium metal from magnets, I've started investigations into deep eutectic solvents inspired by this
thread and some additional searching. You can find my latest progress on this project over at the neodymium thread, here: http://www.sciencemadness.org/talk/viewthread.php?tid=14145&...
====================
The liquid I wanted to start with was choline chloride / urea at a 1:2 mole ratio. I purchased both chemicals from eBay - the choline chloride (CC) as
fairly damp transparent crystals, and the urea in prill form. The CC has a rather strong, disagreeable smell to it that reminds me of triethylamine. I
didn't bother drying it this time, but I put a portion in a dessicator for future use.
I ground 8.0g urea down to a powder in a mortar and pestle, and loaded this into a screw-top test tube. I then scooped 9.5g CC into the tube on top of
the urea. Stoichiometrically, I only needed 9.3g but I wanted to add a little excess to help account for the water it's obviously absorbed.
This setup initially looked like this:

I then took this outside and gently heated with a propane torch, moving the tube in and out of the flame to reduce hot spots and prevent any
decomposition. In retrospect, I should have put the urea on top of the CC instead since it has a lower melting point. As it was, the urea melted first
and I had to slosh it around a bit to dissolve the CC sitting on top of it. Once everything was liquid, I stopped heating.
After cooling, an interesting thing happened: two layers of crystallization!

You can see it had solidified on the top and bottom, but left a small band of liquid in the middle. I suspected that this happened because I did not
mix the liquid well enough, and while it formed enough of a mixture to be liquid while hot, it was not close enough to the eutectic point to stay
liquid at room temperature. So the top layer would be CC-rich eutectic and the bottom layer would be urea-rich, while the middle was "just right."
I then took the test tube and heated again until molten. This time I used a glass rod to stir everything around and mix thoroughly. Upon cooling, it
stayed liquid!

The liquid is very viscous, similar to syrup. The little black bits floating around are from when I hit the CC with the torch a little too hard and
burnt a small bit of it.
I look forward to experimenting with electrodeposition of metals with these types of solvents. It sounds like the CC / glycerol mixture may be more
suitable for this, but that's what experimentation is all about. More to come!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very nice indeed.
I'd try to dissolve LiCl into it. Easier to obtain than anh. NdCl<sub>3</sub>, just as a starting point...
|
|
|
Azane
Harmless

Posts: 22
Registered: 9-7-2013
Member Is Offline
Mood: No Mood
|
|
Help with ionic liquids
So I've recently become interested in ionic liquids (mainly for applications regarding water-sensitive solutes), and I have a few questions.
According to a video I saw on youtube, some of them can dissolve transition metal oxides, which are normally insoluble due to the electrophilic nature
of transition metal ions. My first concern is how strongly basic the oxide ions of transition metal oxides would be in solution. I'm worried about
that because, at the moment, the only ionic liquid I can make, with the materials to which I have access, is ethanaminium nitrate, which would
probably react with the oxide ions to form water, ethanamine, and a metal nitrate, or so I assume, considering that ammonium ions can be deprotonated
by hydroxide ions (more weakly basic than oxide ions) to form water and ammonia.
And if water-insoluble oxides are soluble in ionic liquids, would other normally insoluble/water sensitive ions behave similarly, assuming that the
solvent ionic liquid does not feature portions that are susceptible to reduction/deprotonation? For instance, could sulfide, nitride, imide, amide,
phosphide, or ethynediide (acetylide) dissolve without reacting? Furthermore, could alkali and alkaline earth metals dissolve in ionic liquids, just
as they do in anhydrous ammonia?
I am also wondering if there are other ionic liquids I could make. The various cations I've focused on are those formed by dimethylamine, ethanamine,
2-aminoethanol, 1,2-diaminoethane, and the methylated derivatives thereof. (All in all, that totals out to 28 unique cations, including those derived
from the non-methylated precursors, except for those of dimethylamine, which would be methanamine and ammonia). Of these, I think that
1,2-diaminoethane and its derivatives could have the greatest likelihood of forming ionic liquids with the proper anion.
The anions I've considered include nitrate, sulfate, disulfate, dithionate, 2-hydroxyethanoate (glycolate), 2-aminoethanoate (glycinate), and
2-aminoethanesulfonate (taurate). Of these, I think that 2-aminoethanesulfonate could most likely form ionic-liquids.
Confirm or repudiate whatever speculation of mine that you can, and give any additional information you think could help, please.
Thanks.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Allow me to direct you to the ionic liquid (deep eutectic solvent) thread that I resurrected a few days ago, located just a few posts below this one:
http://www.sciencemadness.org/talk/viewthread.php?tid=10529
The thread itself may not answer your questions directly, but there are numerous links posted that may offer better insight. In any case, that would
be the appropriate thread to post these questions in rather than starting a new one.
I just started exploring these solvents, so I do not have any answers myself as of yet.
|
|
|
Azane
Harmless

Posts: 22
Registered: 9-7-2013
Member Is Offline
Mood: No Mood
|
|
Cool, thanks man.
|
|
|
bfesser
|
Threads Merged
28-1-2014 at 16:03 |
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
An interesting development: After making this a week ago and letting it sit in my lab, lots of needle crystals have formed in the liquid.

Perhaps I didn't get the ratio of the components quite right?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | An interesting development: After making this a week ago and letting it sit in my lab, lots of needle crystals have formed in the liquid.
Perhaps I didn't get the ratio of the components quite right? |
If so, the clear liquid probably is the eutectic.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I made another batch of the eutectic yesterday, this time with choline chloride dried for a week or so over calcium chloride. I used 8.0g urea and
9.3g dry(ish) CC. I also mixed the two reagents before heating, which made things go a bit more smoothly. They've melted together nicely, but left a
lot of tiny white specks throughout the liquid. I can't tell if these are bubbles or undissolved reactants - the viscousity of the liquid may be
trapping the tiny bubbles from moving, if that is what they are. I'll observe this batch and see if any needles separate out. I'm hopeful that since I
dried the CC I can get it closer to the eutectic point.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
I've experimented with deep eutectic solvents as well, I've made some new DES of my own , can you tell me who sells choline chloride on ebay I can't
find any.
[Edited on 7-4-2015 by gatosgr]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I bought it from user adpy-chemistry. He doesn't have any items for sale currently, and if there isn't anything else on eBay right now then I don't
know what to tell you. Just keep checking every now and then and everything shows up eventually!
Now that it's been over a year, I suppose I should update on what happened to the liquid from my last post  It ended up clearing nicely and has remained liquid this whole time. So it looks like drying the choline chloride and
intimately mixing the reagents before melting them together is the way to go. It ended up clearing nicely and has remained liquid this whole time. So it looks like drying the choline chloride and
intimately mixing the reagents before melting them together is the way to go.
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am interested in this. I first heard of ionic liquids 9months ago. An extremely large number of possible applications. I will post a list when I get
back to civilisation.
I had assumed that this was going to be out of reach as a home chemist. Now you tell me that it enters the realm of the possible and I am intrigued.
J.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Actually the problem with water being in the DES is that it becomes part of the DES in stoichiometric values and it will not evaporate , there's a
research about that. Well I follow this guideline for making a DES : the uglier the molecules are the most likely they are to form a DES. Theres also
correlation between the hydrogen bond donors and the DES strength. I have a hard time sourcing materials are there any online shops that sell
chemicals except ebay?
[Edited on 8-4-2015 by gatosgr]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Choline chloride is sold by the tanker-load to large commercial poultry farms... the battery chicken variety. I got 25l of 70% solution for free by
doing a bit of phoning around and then getting them to tap 'a little' for me from the pipes after a tanker delivery.
The solution can be boiled outside in a skillet on a gas stove to dry until it's really viscous. Cooling down then forms a mass of crystalline
material that should be sealed while still hot to prevent it from absorbing atmospheric water. This cooking also drives off fishy smelling impurities
(I assume trimethylamine and friends?) so that one is left with pretty decent material.
I've had LOTS of fun with this including making choline soap (reported somewhere here) and yes, prepared many DES's as well.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
I think you mean 25ml right? How pure did it get with rectystallization?
[Edited on 8-4-2015 by gatosgr]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
No, it was 25 litres! I had to buy and bring my own large 25l container for it. I arranged it beforehand with the people importing the material, I
told them I was trying to make a new kind of choline soap with it and they were happy to supply it for free. They said I could simply tap what's left
in at the bottom of a large storage containers and piping.
I did not analyse it for purity, the original material was feed grade, so I assume that's pretty pure. It has traces of fishy smelling compounds, but
these are volatile, so they came off during the boiling down. There was also a small amount of orange-brown turbidity which I attributed to rust from
tank storage. This settled to the bottom of my container over a couple of weeks so that the top liquid was completely transparent and could be
decanted (the settling was slow because the liquid's viscous).
The recrystallized, slightly tacky white crystals, were odourless. All-in-all, I was very pleased with it all and it gave excellent results in a wide
range of experiments I did with it.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
You have pretty generous poultry farmers in your region. 
ChCl although very cheap is almost impossible to get a hold of...does anybody know a substitute?
[Edited on 9-4-2015 by gatosgr]
|
|
|
DeIonizedPlasma
Harmless

Posts: 21
Registered: 28-9-2014
Member Is Offline
Mood: No Mood
|
|
I have been interested as well in deep eutectic solvents lately, and managed to devise a way that I believe made choline chloride. Because of
the difficulty everyone seemed to be having obtaining choline chloride, I looked for another choline salt with an anion that would form as insoluble a
precipitate as possible when combined with a halide salt. In the end I arrived at choline bitartrate, which is much easier to find as a supplement. I
dissolved 0.2 mol of the bitartrate in 500mL of water (0.2 moles was chosen semi-arbitrarily, IIRC it was very close to a saturated solution in 500mL
and I wanted to make a small batch as a test for the first time) and a saturated solution of 0.2 moles potassium chloride. Potassium bitartrate is
nearly insoluble in water, making it perfect for the isolation of the choline chloride.
I mixed the two solutions and within a few seconds, small crystals began precipitating which at first I thought were bubbles due to their size (They
looked just like a carbonated beverage, but instead of rising they fell). I stirred the solution and it immediately turned completely white and
opaque, precipitating vigorously. By the end there was about 3/4 inch of precipitate on the bottom of the 1L beaker, which would seem to agree with
the vast insolubility of potassium bitartrate. I filtered this and got a large amount of clear fluid which held the ChCl.
To verify that I had ChCl, I put a few mL on a watch glass and put that on a hot plate. As it boiled, a white flakey salt was deposited on the edge of
the glass. By the time it had almost boiled completely off, the edges were beginning to brown and blacken which would seem to confirm an organic salt.
Once completely dry, I left the watch glass out and waited to see if it would deliquesce. I waited about 20 minutes and did not see anything
happening, so I left it out overnight. When I came back the next day it had clearly deliquesced, and there were a few tiny grains of what appeared to
be a salt scattered in the liquid.
At this point I am planning on dehydrating the majority of the solution and running tests for purity to see if I have something I can work with. The
Potassium bitartrate is still very wet, which I assume is due to the choline chloride being mixed in even after filtration and holding moisture in the
mass. If I can manage to dry this I will measure it to get an approximate value of yield.
Does anyone have suggestions for dehydrating a deliquescent salt? I am currently looking into the acetone method but would be interested in seeing
what else you guys can come up with.
|
|
|
| Pages:
1
2
3
4
5 |