| Pages:
1
..
16
17
18
19
20
..
77 |
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
well, here is a "purty pic" to balance out yer sin kiddo LMFAO. Freshly distilled clove oil:

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
These crystals formed from an unsuccessful reaction where I got back the compound what I started from. However, they still look great!
I collected the best picture from crystals from my blog and published them at Béhance, check it out: Béhance
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by The Volatile Chemist  | | LOL arkoma. Nice pictures though. What about diluted sulfuric acid? I just want to get rid of the pink-red dye. I tried to make HCl by reaction of
H2SO4 and Calcium carbonate. I think I added too much sulfuric acid (I diluted it to 8.8 mol/L approx) at too low concentration.
I think it might have made *some* HCl, but it still made some calcium sulfate when mixed with Ca(OH)2. It also turned the pink-red dye
yellow. I must have chlorinated the dye, lol. |
How do you make HCl with sulfuric acid and calcium carbonate? There's not a single atom of chlorine in that entire reaction!
Also, can't you just buy some decent HCl at your local hardware store? They have it near the pool stuff usually, and it's a lot easier than making it,
unless of course you're just trying to for the fun of it.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
LOL, ya can't^^
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Quote: Originally posted by kristofvagyok  |
These crystals formed from an unsuccessful reaction where I got back the compound what I started from. However, they still look great!
I collected the best picture from crystals from my blog and published them at Béhance, check it out: Béhance |
Thanks for the beautiful pictures, I always enjoy them
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|

Raw eugenol. Distilled from 24 gms whole cloves. Still surprising me that some of these organics are denser than water.

| Quote: | Formula: C10H12O2
Boiling point: 489.2°F (254°C)
Molar mass: 164.2 g/mol
Melting point: 18.5°F (-7.5°C)
Density: 1.06 g/cm³
IUPAC ID: 4-Allyl-2-methoxyphenol |
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by zts16  | Quote: Originally posted by The Volatile Chemist  | | LOL arkoma. Nice pictures though. What about diluted sulfuric acid? I just want to get rid of the pink-red dye. I tried to make HCl by reaction of
H2SO4 and Calcium carbonate. I think I added too much sulfuric acid (I diluted it to 8.8 mol/L approx) at too low concentration.
I think it might have made *some* HCl, but it still made some calcium sulfate when mixed with Ca(OH)2. It also turned the pink-red dye
yellow. I must have chlorinated the dye, lol. |
How do you make HCl with sulfuric acid and calcium carbonate? There's not a single atom of chlorine in that entire reaction!
Also, can't you just buy some decent HCl at your local hardware store? They have it near the pool stuff usually, and it's a lot easier than making it,
unless of course you're just trying to for the fun of it. |
LOL, oops, meant CaCl2.......
Edit: And yea, just for fun. If I can make it, it's more rewarding then buying it, even if it isn't better quality stuff.
[Edited on 7-14-2014 by The Volatile Chemist]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Synthesis of 8-bromocaffeine. Copious amounts of bromine were produced, fun!

|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
about .5gm ethylvanillin, courtesy of my new sep funnel

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Very nice picture Mailinmypocket!
Do you have or know any practical use for bromo-caffeine? I have a lot of caffeine and no practical use for it, and the means to make the bromo
variant out of it.
I found it promotes aldoximes to nitriles (Sigma), but nothing really comes to mind still.
Do you have any means to test the conversion of the caffeine? (assuming you started from caffeine).
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
arkoma, is this from one of your steam distillations?
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
No, I got a nifty little 125ml pyrex sep funnel yesterday in a gift box---and had about 60ml of diethyl ether squirreled away. Extracted it from "el
cheapo" kroger artificial vanilla flavoring. Ethylvanillin was listed as the flavorant. It's weird cause it smells kind of "peppery" to me.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | Very nice picture Mailinmypocket!
Do you have or know any practical use for bromo-caffeine? I have a lot of caffeine and no practical use for it, and the means to make the bromo
variant out of it.
I found it promotes aldoximes to nitriles (Sigma), but nothing really comes to mind still.
Do you have any means to test the conversion of the caffeine? (assuming you started from caffeine). |
Thank you 
As far as making 8-bromocaffeine I dont have any practical uses for it. Mainly I was just desperate to do something with the 100g of caffeine powder I
have had kicking around for years. For checking purity I will depend on the m.p. - I have recrystallized the product from ethanol and am waiting on
the beautiful crystals to dry and will do a mp on it afterwards to see what the outcome was.
I got the procedure from this document:
Attachment: bromocaffeine.pdf (745kB)
This file has been downloaded 517 times
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Mailinmypocket  | Quote: Originally posted by Tsjerk  | Very nice picture Mailinmypocket!
Do you have or know any practical use for bromo-caffeine? I have a lot of caffeine and no practical use for it, and the means to make the bromo
variant out of it.
I found it promotes aldoximes to nitriles (Sigma), but nothing really comes to mind still.
Do you have any means to test the conversion of the caffeine? (assuming you started from caffeine). |
Thank you 
As far as making 8-bromocaffeine I dont have any practical uses for it. Mainly I was just desperate to do something with the 100g of caffeine powder I
have had kicking around for years. For checking purity I will depend on the m.p. - I have recrystallized the product from ethanol and am waiting on
the beautiful crystals to dry and will do a mp on it afterwards to see what the outcome was.
I got the procedure from this document:
|
I apologize for not saying it as well when I first saw it, but that is a very nice picture.
This might be an interesting project that could use your 8-bromocaffeine.
Basic Caffeine Derivatives
[Edited on 18-7-2014 by Loptr]
[Edited on 18-7-2014 by Loptr]
Attachment: US2688618.pdf (218kB)
This file has been downloaded 508 times
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice document- thanks! Next will be 8-chlorocaffeine I suppose 
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Here's some more reading on caffeine.
Attachment: phpG7uNGe (66kB)
This file has been downloaded 507 times
Attachment: phpwznInc (98kB)
This file has been downloaded 543 times
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
You will have to append .pdf to the end of those files.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
| Quote: | | You will have to append .pdf to the end of those files. |
not if ya got penguins "under the hood" 
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by arkoma  | | Quote: | | You will have to append .pdf to the end of those files. |
not if ya got penguins "under the hood"  |
I started to specifically mention Windows, in the event someone downloaded and couldn't open, they would have those instructions. I am at work and
software engineer by trade. I am currently running Windows 7 and a headless VM of CentOS, so I am using Windows to view the PDFs at this time. At
home, it's back to the MBP. 
[Edited on 18-7-2014 by Loptr]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
The Arizona sun backlighting some Copper Acetate

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Wow, that's a cool picture
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
https://www.youtube.com/watch?v=Htf1YupWCg4&feature=yout...
Crashing piperine out of solution--think I got it right this time. Hopefully have xtals next day or so

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
How much pepper did you start with?
|
|
|
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
An alloy of Al+Cu :-) under SEM
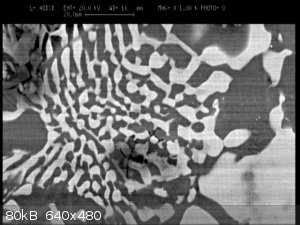
|
|
|
wish i had a kraken!!!
Hazard to Others
  
Posts: 157
Registered: 22-3-2012
Member Is Offline
Mood: No Mood
|
|
Candy Rocket fuel ! (Sorbitol + KNO3)

|
|
|
| Pages:
1
..
16
17
18
19
20
..
77 |