| Pages:
1
..
16
17
18
19
20
..
23 |
katyushaslab
Hazard to Self
 
Posts: 81
Registered: 19-1-2021
Member Is Offline
Mood: precipitating
|
|
For the second paper you linked, the starting material (4-Amino-1,2,5-oxadiazole-3-carbonitrile) seems to be the hardest part of the problem (to me).
Oxidation of the amino group to a nitro group might be doable with KMnO4 under basic conditions, then I think its a condensation under basic
conditions? These two steps might even be doable in one pot. It reminds somewhat of the 5-aminotetrazole to the Sodium 5,5 nitrotetrazole procedure.
The remaining steps (forming the tetrazole rings with sodium azide, and then forming the complex) don't look terribly difficult.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I found another paper (attached) that describes their improved synthesis of a number of key materials. They make
4-Amino-1,2,5-oxadiazole-3-carbonitrile in two steps from malononitrile. In many of the papers I have read on linked furazans, malononitrile seems to
be the starting material...
Attachment: MULTI-CYCLIC OXADIAZOLES.pdf (1.4MB)
This file has been downloaded 395 times
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | In my research into triazoles, I recently stumbled onto a class of coordination complexes known as EMOFs - energetic metal-organic frameworks.
In a number of very new papers, the authors are throwing around some quite extraordinary numbers:
Substances having measured sensitivities less than TNT coupled with calculated detonation energy of 3.9 kcal/g (that is not an error; about twice ONC
or CL-20), Pcj of 50-60 GPa and VOD of 10-11 km/s. In addition, they have measured thermal stabilities of around 300C.
Of course, several of these numbers are calculated and it remains to be seen whether the models are any good at predicting performance at these
extremes of the spectrum. Still, if they are reasonably close, it is a huge leap in performance, without any compromise with regards to safety.
Some of the most energetic EMOFs I have seen are based on coupled furazan-tetrazoles as ligands around metal ions. These form 1-,2- , or 3-D
frameworks that can have other ions in the voids in the structure.
Some appetizers:
https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b01636#
Almost 12 km/s
Very high densities
[Edited on 10-1-2022 by Microtek]
[Edited on 10-1-2022 by Microtek] |
Have any of these been actually made and tested? Are these doable for you to synth?
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Most of these have been synthesized and tested with respect to density, heat of formation, stability and sensitivity. Most of the energetic properties
are computed via semi-empirical methods such as the Kamlet-Jacobs equations. That is why I say that it remains to be seen whether these equations work
for this kind of material.
I believe that I can relatively easily prepare the material called ATRZ-1 ([Cu(4,4'-azo-1,2,4-triazole)3(NO3)2]n). It is basically made by mixing
boiling aqueous solutions of Cu(NO3)2 and 4,4'-azo-1,2,4-triazole, filtering and allowing the filtrate to slowly evaporate until crystals form. The
ligand 4,4'-azo-1,2,4-triazole is prepared by oxidative azo-coupling of 4-amino-1,2,4-triazole using sodium dichloroisocyanurate (pool chemical). As
mentioned previously, I possess 4-amino-1,2,4-triazole from an old experiment, and I have prepared 4,4'-azo-1,2,4-triazole (I hope). I will attempt
the synthesis of ATRZ-1, and also see what happens if Cu(ClO4)2 is substituted for the nitrate.
ATRZ-1 has a heat of detonation of more than 15 kJ/g, but a density of only 1.68 g/cc, so the performance is predicted to be only about the level of
HMX, but a perchlorate analogue might be better.
On another note, I received my carbohydrazide recently, and have prepared urazine following the procedure from Inorganic Synthesis vol 4. It involves
adding concentrated HCl and then slowly heating to 220C. Then water is added to dissolve the byproduct hydrazine*HCl, and the insoluble urazine is
filtered off. The yield exactly matched the litterature procedure. I then added a slight excess of sulfuric acid to the recovered filtrate, which
caused a large amount of white crystals to precipitate. This should be hydrazine sulfate, and after filtering, washing with cold water and drying, I
recovered a yield of about 70%. In total, 23.4 g carbohydrazide was converted to 11.5 g urazine and 15.6 g hydrazine sulfate.
The 1.16 g urazine was then reacted with one molar equivalent of 50% HClO4, and stirred for 1 hour at room temp, then 1 hour at 50C. Then the water
was evaporated by placing the entire mass in a petri dish and heating to 60C on the hotplate. The product UZP is very dense and extremely energetic
(in the hammer test), but moderately hygroscopic. I tried desensitizing it by dissolving 5 parts by weight of bees wax in a minimal amount of
detergent gasoline, mixing this with 95 parts of the UZP and evaporating the gasoline while continually agitating the mix. The sensitivity is indeed
reduced a little, but the wax does nothing for the hygroscopicity. The powder was pressed into a 7mm ID brass tube and easily achieved a packing
density of 2.05 g/cc, however, when this was tested in my usual setup, a very disappointing result of 2.92 mm dent depth in 20 mm Al bar stock was
achieved.
For comparison:
Picric acid: 3.53 mm
5-ATz*NO3: 3.84 mm
RDX: 4.13 mm
HMX: 4.35 mm
It was only at this point I discovered the hygroscopicity issue. I had left weighing boats with both some of the neat crystals and also some of the
wax treated powder in my workshop for testing. The relative humidity is slightly higher in my workshop than in my lab, and the samples that were in my
lab *looked* fine, but the ones in the workshop had visibly absorbed atmospheric moisture. It may certainly be possible to work around both the
sensitivity issue and the hygroscopicity (the right PBX formulation may solve both), but before working on that, I will conduct further tests to see
if better performance can't be achieved from the dry product.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
You use plate dent test with aluminium brick I estimate. Density 2.05 seems good. ID 7mm OK. But weight of output segment? 300mg? 500mg?
Maybe I missed it. But I don't see the weight of the output segment in the text.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | Most of these have been synthesized and tested with respect to density, heat of formation, stability and sensitivity. Most of the energetic properties
are computed via semi-empirical methods such as the Kamlet-Jacobs equations. That is why I say that it remains to be seen whether these equations work
for this kind of material.
I believe that I can relatively easily prepare the material called ATRZ-1 ([Cu(4,4'-azo-1,2,4-triazole)3(NO3)2]n). It is basically made by mixing
boiling aqueous solutions of Cu(NO3)2 and 4,4'-azo-1,2,4-triazole, filtering and allowing the filtrate to slowly evaporate until crystals form. The
ligand 4,4'-azo-1,2,4-triazole is prepared by oxidative azo-coupling of 4-amino-1,2,4-triazole using sodium dichloroisocyanurate (pool chemical). As
mentioned previously, I possess 4-amino-1,2,4-triazole from an old experiment, and I have prepared 4,4'-azo-1,2,4-triazole (I hope). I will attempt
the synthesis of ATRZ-1, and also see what happens if Cu(ClO4)2 is substituted for the nitrate.
ATRZ-1 has a heat of detonation of more than 15 kJ/g, but a density of only 1.68 g/cc, so the performance is predicted to be only about the level of
HMX, but a perchlorate analogue might be better.
On another note, I received my carbohydrazide recently, and have prepared urazine following the procedure from Inorganic Synthesis vol 4. It involves
adding concentrated HCl and then slowly heating to 220C. Then water is added to dissolve the byproduct hydrazine*HCl, and the insoluble urazine is
filtered off. The yield exactly matched the litterature procedure. I then added a slight excess of sulfuric acid to the recovered filtrate, which
caused a large amount of white crystals to precipitate. This should be hydrazine sulfate, and after filtering, washing with cold water and drying, I
recovered a yield of about 70%. In total, 23.4 g carbohydrazide was converted to 11.5 g urazine and 15.6 g hydrazine sulfate.
The 1.16 g urazine was then reacted with one molar equivalent of 50% HClO4, and stirred for 1 hour at room temp, then 1 hour at 50C. Then the water
was evaporated by placing the entire mass in a petri dish and heating to 60C on the hotplate. The product UZP is very dense and extremely energetic
(in the hammer test), but moderately hygroscopic. I tried desensitizing it by dissolving 5 parts by weight of bees wax in a minimal amount of
detergent gasoline, mixing this with 95 parts of the UZP and evaporating the gasoline while continually agitating the mix. The sensitivity is indeed
reduced a little, but the wax does nothing for the hygroscopicity. The powder was pressed into a 7mm ID brass tube and easily achieved a packing
density of 2.05 g/cc, however, when this was tested in my usual setup, a very disappointing result of 2.92 mm dent depth in 20 mm Al bar stock was
achieved.
For comparison:
Picric acid: 3.53 mm
5-ATz*NO3: 3.84 mm
RDX: 4.13 mm
HMX: 4.35 mm
It was only at this point I discovered the hygroscopicity issue. I had left weighing boats with both some of the neat crystals and also some of the
wax treated powder in my workshop for testing. The relative humidity is slightly higher in my workshop than in my lab, and the samples that were in my
lab *looked* fine, but the ones in the workshop had visibly absorbed atmospheric moisture. It may certainly be possible to work around both the
sensitivity issue and the hygroscopicity (the right PBX formulation may solve both), but before working on that, I will conduct further tests to see
if better performance can't be achieved from the dry product. |
Please! This is a project I am greatly interested in! How sensitive was this? I assume similar to SADS or so? Amazing that water desensitizes it so
much. Perhaps the nitrate analog might be almost as interesting? I am after performance at any cost… and this seems like the king other than the
EMOFs. If that synth works out maybe we can find a way to synth the ones at 2-3g/cc. They hold more promise than Urazine but the difficult synthesis
seems depressing.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | Most of these have been synthesized and tested with respect to density, heat of formation, stability and sensitivity. Most of the energetic properties
are computed via semi-empirical methods such as the Kamlet-Jacobs equations. That is why I say that it remains to be seen whether these equations work
for this kind of material.
I believe that I can relatively easily prepare the material called ATRZ-1 ([Cu(4,4'-azo-1,2,4-triazole)3(NO3)2]n). It is basically made by mixing
boiling aqueous solutions of Cu(NO3)2 and 4,4'-azo-1,2,4-triazole, filtering and allowing the filtrate to slowly evaporate until crystals form. The
ligand 4,4'-azo-1,2,4-triazole is prepared by oxidative azo-coupling of 4-amino-1,2,4-triazole using sodium dichloroisocyanurate (pool chemical). As
mentioned previously, I possess 4-amino-1,2,4-triazole from an old experiment, and I have prepared 4,4'-azo-1,2,4-triazole (I hope). I will attempt
the synthesis of ATRZ-1, and also see what happens if Cu(ClO4)2 is substituted for the nitrate.
ATRZ-1 has a heat of detonation of more than 15 kJ/g, but a density of only 1.68 g/cc, so the performance is predicted to be only about the level of
HMX, but a perchlorate analogue might be better.
On another note, I received my carbohydrazide recently, and have prepared urazine following the procedure from Inorganic Synthesis vol 4. It involves
adding concentrated HCl and then slowly heating to 220C. Then water is added to dissolve the byproduct hydrazine*HCl, and the insoluble urazine is
filtered off. The yield exactly matched the litterature procedure. I then added a slight excess of sulfuric acid to the recovered filtrate, which
caused a large amount of white crystals to precipitate. This should be hydrazine sulfate, and after filtering, washing with cold water and drying, I
recovered a yield of about 70%. In total, 23.4 g carbohydrazide was converted to 11.5 g urazine and 15.6 g hydrazine sulfate.
The 1.16 g urazine was then reacted with one molar equivalent of 50% HClO4, and stirred for 1 hour at room temp, then 1 hour at 50C. Then the water
was evaporated by placing the entire mass in a petri dish and heating to 60C on the hotplate. The product UZP is very dense and extremely energetic
(in the hammer test), but moderately hygroscopic. I tried desensitizing it by dissolving 5 parts by weight of bees wax in a minimal amount of
detergent gasoline, mixing this with 95 parts of the UZP and evaporating the gasoline while continually agitating the mix. The sensitivity is indeed
reduced a little, but the wax does nothing for the hygroscopicity. The powder was pressed into a 7mm ID brass tube and easily achieved a packing
density of 2.05 g/cc, however, when this was tested in my usual setup, a very disappointing result of 2.92 mm dent depth in 20 mm Al bar stock was
achieved.
For comparison:
Picric acid: 3.53 mm
5-ATz*NO3: 3.84 mm
RDX: 4.13 mm
HMX: 4.35 mm
It was only at this point I discovered the hygroscopicity issue. I had left weighing boats with both some of the neat crystals and also some of the
wax treated powder in my workshop for testing. The relative humidity is slightly higher in my workshop than in my lab, and the samples that were in my
lab *looked* fine, but the ones in the workshop had visibly absorbed atmospheric moisture. It may certainly be possible to work around both the
sensitivity issue and the hygroscopicity (the right PBX formulation may solve both), but before working on that, I will conduct further tests to see
if better performance can't be achieved from the dry product. |
Dornier could compute one or two of these to verify. His EOS is quite different
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
LL: My standard test setup is as follows:
A 3D-printed holder is glued to the 20mm thick aluminum bar stock. Then the brass tube (7mm ID, 8mm OD, 40mm length, and the end is cut on my lathe to
ensure that it is completely perpendicular to the axis of the tube) is inserted and glued into the holder.
The brass tube contains 1.000 +- 0.002 g of the substance to be tested and 0.300 +- 0.002 g PETN. It is initiated by aminoguanidinium nickel
perchlorate.
MineMan
UZP is quite sensitive when dry, about on the level of ETN. With wax desensitization, maybe on par with PETN.
I just did another test with a fresh batch of UZP. Thia time I stored the powder in a dessicator, then prepared a PBX consisting of 6 parts PIB/5w40
oil (both dissolved in detergent gasoline) and 94 parts UZP. After mixing the components, most of the gasoline was evaporated, and the almost dry
composite was placed in the dessicator overnight. Then it was pressed into the brass tube at a quite moderate pressure, to eliminate potential dead
pressing as an issue. The density of the charge was still right around 2.00 g/cc, and the assembled charge was placed back into the dessicator until
just before firing.
Despite all this, the result was only 2.934 mm dent depth. I therefore conclude that either it is impractical to keep the material dry enough, or else
the problem lies elsewhere. Perhaps UZP loses more performance at this scale than some other energetics like RDX and HMX, or perhaps the model simply
overestimates the performance of this material. Perhaps it is very easily dead pressed.

|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Great measurement, hat off. I finally see someone doing accurate tests. Thanks.....
And not like some who wrote "I heard it exploded", sure it works...
[Edited on 17-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | LL: My standard test setup is as follows:
A 3D-printed holder is glued to the 20mm thick aluminum bar stock. Then the brass tube (7mm ID, 8mm OD, 40mm length, and the end is cut on my lathe to
ensure that it is completely perpendicular to the axis of the tube) is inserted and glued into the holder.
The brass tube contains 1.000 +- 0.002 g of the substance to be tested and 0.300 +- 0.002 g PETN. It is initiated by aminoguanidinium nickel
perchlorate.
MineMan
UZP is quite sensitive when dry, about on the level of ETN. With wax desensitization, maybe on par with PETN.
I just did another test with a fresh batch of UZP. Thia time I stored the powder in a dessicator, then prepared a PBX consisting of 6 parts PIB/5w40
oil (both dissolved in detergent gasoline) and 94 parts UZP. After mixing the components, most of the gasoline was evaporated, and the almost dry
composite was placed in the dessicator overnight. Then it was pressed into the brass tube at a quite moderate pressure, to eliminate potential dead
pressing as an issue. The density of the charge was still right around 2.00 g/cc, and the assembled charge was placed back into the dessicator until
just before firing.
Despite all this, the result was only 2.934 mm dent depth. I therefore conclude that either it is impractical to keep the material dry enough, or else
the problem lies elsewhere. Perhaps UZP loses more performance at this scale than some other energetics like RDX and HMX, or perhaps the model simply
overestimates the performance of this material. Perhaps it is very easily dead pressed. |
Excellent work! Have you given up with this material or would you be willing to do a test at 1.9 density to avoid deadpressing? I admit the results
seem disappointing. But we do know, at the very least it should be at least as powerful as RDX. You noticed that the hammer test was extremely
energetic. Is it possible to seal the crystals from moisture during recrystallization with an additive?
Can you try making a mix of 50 percent PETN and 50 percent Urazine? Then we could access forsure, if this is weaker or stronger than PETN. I have a
feeling it’s not a full det. No compound at that density would produce such a small dent
What size were your crystals? The paper reported .5-1mm
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
No, I haven't given completely up on it, and I do have some left of my first batch of urazine. I was thinking that I would see if the nitrate is
useful, and I could also try a PETN mix and maybe a very lightly pressed charge. I tried making the picrate, but adding urazine to molten picric acid
did not produce anything other than the starting materials.
My UZP crystals were smaller than the ones reported in the paper, but still relatively large. They are not uniform and they don't crystallize cleanly.
Since the material is produced by heating the mix of HClO4 and urazine, I agitate it regularly to avoid a solid lump of highly sensitive and energetic
HE that I then need to break down mechanically. When I triturate the crystals under detergent gasoline, they are easily reduced in size without
applying too much force.
I would like to try a charge without any additives, but am leery of pressing such a sensitive HE (especially as the crystals "creak" as they are
pressed, somewhat like snow when it is compacted).
On another note, I did some more experiments with 4-amino-1,2,4-triazolium perchlorate. I prepared some from equimolar (10 mmol) amounts of perchloric
acid and the parent triazole. Then I evaporated the water by heating in a petri dish on a hotplate. I let it coodown with agitation and then placed
it in a dessicator. After sitting ca. 24 hours, I ground it in a motar and pestle, about 30 mg at a time. Then I placed it in the dessicator again.
1.000 g of the, now much finer, powder was pressed uneventfully to 1.75 g/cc which corresponds to more than 96 % TMD according to a paper that I found
(attached).
On detonation, the dent produced was 4.38 mm deep, which is on par with HMX (4.35 mm). In the paper, they find a sensitivity of 30 kg*cm (HMX 34
kg*cm), but in my own tests, my impression is that it is more sensitive than that (perhaps it is more sensitive to friction which plays a part in most
non-ideal impacts).
Anyway, I also discovered that it melts below 100C. In the paper they say 84C, my own melting point determination says 78C at 12 degrees per minute,
but this may be due to included water. When I let the temp continue to rise, no visible sign of decomposition could be detected up to 225C. This long
molten temperature range makes it a candidate for melt casting, possibly in conjunction with other materials such as the nitrate or maybe urazine
perchlorate. I would still like to lower the sensitivity a little, though.
[Edited on 18-1-2022 by Microtek]
[Edited on 18-1-2022 by Microtek]
Attachment: phpZEHUYt (114kB)
This file has been downloaded 294 times
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | In my research into triazoles, I recently stumbled onto a class of coordination complexes known as EMOFs - energetic metal-organic frameworks.
In a number of very new papers, the authors are throwing around some quite extraordinary numbers:
Substances having measured sensitivities less than TNT coupled with calculated detonation energy of 3.9 kcal/g (that is not an error; about twice ONC
or CL-20), Pcj of 50-60 GPa and VOD of 10-11 km/s. In addition, they have measured thermal stabilities of around 300C.
Of course, several of these numbers are calculated and it remains to be seen whether the models are any good at predicting performance at these
extremes of the spectrum. Still, if they are reasonably close, it is a huge leap in performance, without any compromise with regards to safety.
Some of the most energetic EMOFs I have seen are based on coupled furazan-tetrazoles as ligands around metal ions. These form 1-,2- , or 3-D
frameworks that can have other ions in the voids in the structure.
Some appetizers:
https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b01636#
Almost 12 km/s
Very high densities
[Edited on 10-1-2022 by Microtek]
[Edited on 10-1-2022 by Microtek] |
Those incredible claims caught my eye and I skimmed through the three articles you linked.
First off, 3.9 kcal/g or 16 kJ/g is obviously an error or simply clickbait. A hypothetical "ultimate" explosive in the form of (N-N-)n
decomposing into n N2 would have a detonation energy of about 22 kJ/g, and another in the form of monatomic oxygen and carbon
would have ~20 kJ/g. Anything with reasonable bonds would not come close to 16 kJ/g. ONC and DFF reach about 8 kJ/g and simple tetrazoles around 6
kJ/g. Adding heavy and inert metal atoms will obviously not improve this figure.
In the first article, the authors compare the detonation energy of Cu-btm-NH3OH with RDX and HMX but they botched it completely: their energy is wrong
for the reaction they assume (ammonia as product), and the reaction they assume is wrong. They claim 6.1 kJ/g but the real value is 1.9 g/kJ. They
also brag about their high heat of combustion but that is just a way to say that the oxygen balance is poor...
The second article presents the compound Ag16C54H8N110O27, that is 40% silver by weight, with
an oxygen balance of -31%. The enthalpy of formation is a mediocre 2.3 kJ/g and the density is very low at only 2 g/cm3 for something with
so much silver! Claiming that this should reach nearly 12 km/s is absolutely bonkers. My best guess is 5-6 km/s with a detonation energy of 3 kJ/g.
The problem lies in their choice of method. They use the empirical Kamlet-Jacobs, which was made for CHNO-compounds.
Both article 2 and 3 reference the same paper that applied Kamlet-Jacobs to high nitrogen metal salts and compared the results to EXPLO5 calculations.
The accuracy is acceptable for light metals (less than 1 km/s overestimation) but they don't even evaluate heavy atoms like silver and lead. Lead
azide calculated with an empirical method gives 10.6 km/s and 52 GPa compared to the real 5.2 km/s.
The third article lists a number of EMOFs with high densities and ridiculous detonation performances. Kamlet-Jacobs is once again the problem.
The density increase from added heavy atoms does not improve performance in reality. Lead azide (5.2 km/s) is not better than
hydrazoic acid (7.6 km/s) despite its four times higher density for example. The same applies to tetrazoles and their metal salts and complexes: the
salts improve properties like stability, hygroscopicity etc but not performance.
All papers about EMOFs I can find seem to come from the same Chinese universities, contain the same overestimated detonation performance, and use the
same invalid methods for calculation.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | No, I haven't given completely up on it, and I do have some left of my first batch of urazine. I was thinking that I would see if the nitrate is
useful, and I could also try a PETN mix and maybe a very lightly pressed charge. I tried making the picrate, but adding urazine to molten picric acid
did not produce anything other than the starting materials.
My UZP crystals were smaller than the ones reported in the paper, but still relatively large. They are not uniform and they don't crystallize cleanly.
Since the material is produced by heating the mix of HClO4 and urazine, I agitate it regularly to avoid a solid lump of highly sensitive and energetic
HE that I then need to break down mechanically. When I triturate the crystals under detergent gasoline, they are easily reduced in size without
applying too much force.
I would like to try a charge without any additives, but am leery of pressing such a sensitive HE (especially as the crystals "creak" as they are
pressed, somewhat like snow when it is compacted).
On another note, I did some more experiments with 4-amino-1,2,4-triazolium perchlorate. I prepared some from equimolar (10 mmol) amounts of perchloric
acid and the parent triazole. Then I evaporated the water by heating in a petri dish on a hotplate. I let it coodown with agitation and then placed
it in a dessicator. After sitting ca. 24 hours, I ground it in a motar and pestle, about 30 mg at a time. Then I placed it in the dessicator again.
1.000 g of the, now much finer, powder was pressed uneventfully to 1.75 g/cc which corresponds to more than 96 % TMD according to a paper that I found
(attached).
On detonation, the dent produced was 4.38 mm deep, which is on par with HMX (4.35 mm). In the paper, they find a sensitivity of 30 kg*cm (HMX 34
kg*cm), but in my own tests, my impression is that it is more sensitive than that (perhaps it is more sensitive to friction which plays a part in most
non-ideal impacts).
Anyway, I also discovered that it melts below 100C. In the paper they say 84C, my own melting point determination says 78C at 12 degrees per minute,
but this may be due to included water. When I let the temp continue to rise, no visible sign of decomposition could be detected up to 225C. This long
molten temperature range makes it a candidate for melt casting, possibly in conjunction with other materials such as the nitrate or maybe urazine
perchlorate. I would still like to lower the sensitivity a little, though.
[Edited on 18-1-2022 by Microtek]
[Edited on 18-1-2022 by Microtek] |
Excellent work! Is that the compound that has twice the energy of a regular explosive? Considering it made the same dent as HMX, but at a lower
density, and melt cast! Impressive  . Are there any denser more powerful ones you
can do… well if you decide to after reading dorniers post . Are there any denser more powerful ones you
can do… well if you decide to after reading dorniers post
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Oh, I tend to agree with Dornier. I also noted the inconsistencies and strange mish-mash of models, the fixation on cumbustion energies. Gasoline also
has a great combustion energy (ca. 40 kJ/g), but is not explosive. I also have a hard time seeing where the extra energy would be coming from with
these coordination complexes. However, it only costs me a little work, and a little bit of chemicals that I've got on hand anyway to test some of them
out to know for sure. Sometimes phenomena are observed that doesn't fit with the current understanding. Then, if they are repeatable, that
understanding must be revised. For this reason, the experiment is king, and any theoretical prediction is just a (more or less) educated guess.
Just to clarify, the explosive I was describing in my last post is not one of these exotic EMOFs, it is simply the perchlorate salt of
4-amino-triazole.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Ahh!
Well still impressive for the density. Any luck on the Urazine nitrate or perchlorate
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have tried preparing urazine nitrate, but it seems that the combination of volatility and lower acidity of nitric acid means that mostly (only?)
unreacted urazine is recovered.
I did prepare a 1:1 molar mix of urazine perchlorate and 4-amino-1,2,4-triazole perchlorate by mixing 5 mmol of urazine and 4-ATrz, adding 10 mmol
HClO4 (50%) and evaporating water at 60C. The mix is not quite as powerful as ATrz-perchlorate alone, but more so than a 1:1 mix with PETN. This might
indicate that urazine perchlorate does have some potential, that simply isn't realized under the conditions I have been using. Maybe it requires more
powerful initiation despite being quite sensitive, to perform maximally (like NG which can detonate at different rates depending on initiation and
containment).
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Hmm. Dinitrogen pentaoxide maybe needed?
Do you plan to keep working with the UP… I feel your the only one that can. It’s not overly sensitive so it has great potential.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
N2O5 won't help matters. We don't need the nitronium ion, and the problem is that urazine is so weakly basic that it can't hold on to the nitrate ion.
It doesn't stay protonated, especially since nitric acid is relatively volatile which pushes the UZ*HNO3 <--> UZ + HNO3 equilibrium towards the
right as it evaporates. The same behavior is seen with aminonitroguanidinium nitrate, but to a lesser degree (so it takes longer for ANQN to lose all
the nitrate).
I'm going to try to measure VOD with an oscilloscope, but it is an experimental procedure I haven't tried before (I don't know much about electronics
- I only barely know how to operate an oscilloscope), but I've found some papers that describe different methods.
The simplest method uses ionization probes, which can be made from coaxial cables. It is based on using the conductivity of the reaction zone just
behind the detonation front to act as a switch to close an electrical circuit. This can then be detected with an oscilloscope.
Another option that looks attractive is based on detecting the arrival of the detonation front based on capturing the emitted light, and guiding it to
fast photodiodes using fiberoptic cable. The optical detectors they used in the paper were quite expensive (ca. 500 dollars a piece and I need at
least two - more preferably a few more), but I've found some much more affordable ones that I'm hoping are good enough.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
In my opinion, the detonation pressure is more important than the detonation velocity VoD. For this reason, the dent plate method is the most
convincing. It's cheap, fast and shows the most important things. That is, the mechanical effect on the pad. The speed of light is the highest of all.
But it moves the spacecraft only slightly. Therefore, is best aluminum brick and 1 gram EM. In a column of material that is taller than wider. The
so-called supra-square. In a cavity of 7 mm, for example 15 mm, in a cavity of 8 mm, for example 12 mm. The crater from ETN (or PETN) then serves as a
reference depth. VoD measurement (electrically or optically) has a very laborious preparation, much more grams is used. And the test is loud. Not to
mention the price of the measuring device. In amateur conditions is it luxury. // Let's make things as simple as is possible. But not simplier //
Albert Einstein......
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | N2O5 won't help matters. We don't need the nitronium ion, and the problem is that urazine is so weakly basic that it can't hold on to the nitrate ion.
It doesn't stay protonated, especially since nitric acid is relatively volatile which pushes the UZ*HNO3 <--> UZ + HNO3 equilibrium towards the
right as it evaporates. The same behavior is seen with aminonitroguanidinium nitrate, but to a lesser degree (so it takes longer for ANQN to lose all
the nitrate).
I'm going to try to measure VOD with an oscilloscope, but it is an experimental procedure I haven't tried before (I don't know much about electronics
- I only barely know how to operate an oscilloscope), but I've found some papers that describe different methods.
The simplest method uses ionization probes, which can be made from coaxial cables. It is based on using the conductivity of the reaction zone just
behind the detonation front to act as a switch to close an electrical circuit. This can then be detected with an oscilloscope.
Another option that looks attractive is based on detecting the arrival of the detonation front based on capturing the emitted light, and guiding it to
fast photodiodes using fiberoptic cable. The optical detectors they used in the paper were quite expensive (ca. 500 dollars a piece and I need at
least two - more preferably a few more), but I've found some much more affordable ones that I'm hoping are good enough. |
Microtek! Could lithium work as a good ligand for AQ? For example lithium aminoguanidine diperchlorate? If so, the synthesis should be that of the
copper variant but with lithium instead? Perhaps the lithium could help make these weakly basic molecules more stable? Both nickel and Copper AQ
diperchlorate so remarkable stability outside of water, and no issues with humidity… perhaps the Urazine could be stabilized this way as well… by
adding a metal ligand. Lithium is the most preferable?
LL is right. Detonation pressure only matters.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Lithium (or another metal ion) would be the coordination center, and AQ would be the ligand. This kind of complex is what I use for my primary, though
using nickel instead of lithium. I don't think alkali metals make for very good coordination centers, but coordination chemistry isn't my specialty.
Generally, metal ions do not contribute to the explosive performance (as Dornier pointed out earlier, it's like mixing your wood with ashes before
lighting the fire), but can benefit other parameters such as stability, DDT ability, etc.
It might be possible to make a complex with urazine as a ligand and perchlorate anions around a (transition) metal center. It could then be hoped that
this would make it less sensitive, but more willing to go high order. Then it would be nice if the performance didn't drop too much because of the
metal content.
With regards to my planned VOD measurement, take a look at the paper I'm attaching. They use probe spacings of 1-4mm on a (strongly confined) charge
with rectangular cross section and a width of 0.5 mm. They get measurements with a standard deviation of 13 - 60 m/s, corresponding to an error on the
order of 0.5%. So charge size is not an issue.
It is true that detonation pressure is very important, but there is a very strong correlation between VOD and Pcj, so measuring VOD will give a very
good estimate of Pcj. Additionally, by using several probes, the acceleration of the detonation front could be mapped and this would make it possible
to model things like critical diameter and non-ideal behaviour.
In the attached paper, they use an oscilloscope with 500MHz bandwidth and 2.5 GSa/s, and that kind of specification costs upwards of 10000 dollars,
but if you drop to 200 MHz and 1 GSa/s you can get a very decent one (I'm told) for about 400 dollars. Since 200 MHz should be enough to record 200
oscillations in the time it takes the detonation front to move 10 mm (at 10 km/s), I'm betting on this being sufficient time resolution.
Attachment: Application and Analysis of Discrete Fiber Probes in Determining Detonation Velocity of Microcharges.pdf (3.2MB)
This file has been downloaded 355 times
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | Lithium (or another metal ion) would be the coordination center, and AQ would be the ligand. This kind of complex is what I use for my primary, though
using nickel instead of lithium. I don't think alkali metals make for very good coordination centers, but coordination chemistry isn't my specialty.
Generally, metal ions do not contribute to the explosive performance (as Dornier pointed out earlier, it's like mixing your wood with ashes before
lighting the fire), but can benefit other parameters such as stability, DDT ability, etc.
It might be possible to make a complex with urazine as a ligand and perchlorate anions around a (transition) metal center. It could then be hoped that
this would make it less sensitive, but more willing to go high order. Then it would be nice if the performance didn't drop too much because of the
metal content.
With regards to my planned VOD measurement, take a look at the paper I'm attaching. They use probe spacings of 1-4mm on a (strongly confined) charge
with rectangular cross section and a width of 0.5 mm. They get measurements with a standard deviation of 13 - 60 m/s, corresponding to an error on the
order of 0.5%. So charge size is not an issue.
It is true that detonation pressure is very important, but there is a very strong correlation between VOD and Pcj, so measuring VOD will give a very
good estimate of Pcj. Additionally, by using several probes, the acceleration of the detonation front could be mapped and this would make it possible
to model things like critical diameter and non-ideal behaviour.
In the attached paper, they use an oscilloscope with 500MHz bandwidth and 2.5 GSa/s, and that kind of specification costs upwards of 10000 dollars,
but if you drop to 200 MHz and 1 GSa/s you can get a very decent one (I'm told) for about 400 dollars. Since 200 MHz should be enough to record 200
oscillations in the time it takes the detonation front to move 10 mm (at 10 km/s), I'm betting on this being sufficient time resolution.
|
Your method makes perfect sense after that explanation.
As regards to lithium, it seems it works the best for LLs formula, which was once replaced by copper. Maybe lithium is overlooked? Unfortunately I
don’t understand the chemistry and might be missing something. But if it works to bind the hexamine and perchlorate, why not a better ligand such as
AQ, carbohydrazide, Urazine… and others I am sure. Lithium holds the record for VOd… lithium pentazolate.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
The attached link explains everything. In the last century, a distance between sensors of 10 cm was used. There were no such fast devices for
measurement....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
pdb
Hazard to Others
  
Posts: 100
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
Measuring VOD
Synchronising measuring instruments such as oscilloscopes with such fast phenomena is not easy. Here is an example:
During my engineering studies, I did an internship in an explosives research centre, where I worked on measuring the particle velocity behind the
wavefront, which is of course lower than the VOD, but is nevertheless expressed in km/s. I used the magnetodynamic method, which consists of measuring
the current induced in a metal strip immersed in a magnetic field and entrained by the products of the detonation. The explosive was 200 ml Astrolite
A. Both oscilloscopes were triggered by a probe located a few centimetres upstream of the metal tape, but only one worked, and gave a null image.
Either it was triggered too early or too late, or its calibration was not good. And I didn't get a second chance...
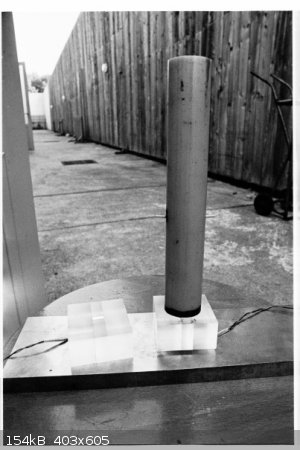         
[Edited on 9-2-22 by pdb]
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, noise and unreliable triggering is why I was thinking that fiberoptics and a fast photodiode might be better. Time will tell. Whether I can get
it to work or not, I'm sure I can find some use for my new oscilloscope.
|
|
|
| Pages:
1
..
16
17
18
19
20
..
23 |
|