| Pages:
1
..
15
16
17
18
19
..
30 |
benzeentje
Harmless

Posts: 17
Registered: 19-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Adas  | Quote: Originally posted by benzeentje  | Hi all, i'm trying to extract caffeine without chloroform or other halogen-alkanes. I have thinner (mostly toluene), terpentine, and white spirit.
Wich one is best, considering this caffeine doesnt need to be edible?
I was thinking thinner (because of the toluene) but it has a boiling point of +-110°C -.- |
Why not to use ether? |
Because i have none. I have acetone too, but this is apperently used as recrystalization solvent instead of extraction solvent as most of the
inpurities in coffee dissolve in acetone too.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Why are English-speaking people almost always saying "sodium metal", "titanium metal", instead of sodium and titanium? I don't see anyone saying
"iron metal" when chemistry is discussed. It's iron, and it's obvious it's a metal.
Is the "_metal" addition required by BE/AE chemical nomenclature (it sure isn't required by the IUPAC standards), or is it a consequence of
proliferation of errors over the Web? I ask that because I've had more than a few brushes with pre-Internet English chemical literature, and such
linguistic constructs are very rare.
Also, the reagent bottles always say stuff like "CALCIUM", and beneath, written in small fonts, is "metallic" or "metal". If it's written in Latin, it
says "metallicum", but always as an additional info. The name of the substance isn't "CALCIUM METAL", it's "CALCIUM".
Since the Web has made a huge boom on the society, constructs such as "CADMIUM METAL" began replacing older, plain and simple "CADMIUM". Why is that?
It sound stupid, at least to me. Reminds me of errors that accumulate and soon become the "truth".
As it's something that is boosted by .com companies that sell compounds online. Sodium has become, from a notoriously difficult to obtain material
which I wanted to own since I saw its photos in elementary school, to a material easy to buy online, for tossing in lakes and toilet bowls by kids
whose chemistry knowledge is scarce. Everytime I hear "sodium metal", I instantly think of those kids.
I've grown up with bottles of "sodium", "natrium", "natrij", "sodio", etc., so yeah, I'm puzzled.
[Edited on 21-3-2012 by Endimion17]
|
|
|
ibro
Harmless

Posts: 14
Registered: 28-3-2012
Member Is Offline
Mood: No Mood
|
|
Does anyone know which gas could be used to boost a car beside N20???
|
|
|
ibro
Harmless

Posts: 14
Registered: 28-3-2012
Member Is Offline
Mood: No Mood
|
|
Can someone tell me a proper way to dispose used engine oil?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.....can someone assist me innaming this compound.......thanks.....solo
C6H5-CH2-CH(COCH3)-CH3
NOte ....i edited because i wrote it wrong....this is now correct!
[Edited on 30-3-2012 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I don't believe that the compound you have written is possible how it is written. In order for a CH-C bond to be possible dangling off of a benzene
ring is if there were to be a double bond. So, C6H5-CH=C(C-CH3)-CH3. Now in order for the C-CH3 to be possible, there would have to be some kind of
triple bond from the other adjacent carbon(not the CH3). Which leads to a hexavalent carbon:
C6H5-CH=C(=-C-CH3)-CH3
___________^^Triple bond (hopefully this posts okay)
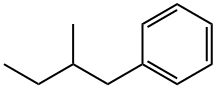
Unless you meant C6H5-CH2-CH(-CH2-CH3)-CH3 ; in which case the name would be (2-methylbutyl)benzene. Unless I am severely misinterperating something
here(entirely likely).
edit : solo I really reccomend checking out ACD labs marvin sketch, it has an awesome IUPAC naming functionality and drawing structures is a breeze.
It works on all operating systems I believe as well and is free with limited functionality.
[Edited on 30-3-2012 by smaerd]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Bring it to some place that will dispose of it. Depends on what country you're in... here in the USA I might bring it to the local Tractor Supply
store. I think (not sure) that any place that sells motor oil has to take back used oil for disposal, up to a certain quantity.
However, assuming that your question is more oriented towards science madness than a practical solution ('How can *I personally* render my used motor
oil environmentally innocuous?'), I might suggest a Babington burner. Google it.
The less you bet, the more you lose when you win.
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by solo  |
.....can someone assist me innaming this compound.......thanks.....solo
C6H5-CH2-CH(COCH3)-CH3
NOte ....i edited because i wrote it wrong....this is now correct!
[Edited on 30-3-2012 by solo] |
Looks like 3-methyl-4-phenylbutan-2-one to me
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Quote: Originally posted by fledarmus  | Quote: Originally posted by solo  |
.....can someone assist me innaming this compound.......thanks.....solo
C6H5-CH2-CH(COCH3)-CH3
NOte ....i edited because i wrote it wrong....this is now correct!
[Edited on 30-3-2012 by solo] |
Looks like 3-methyl-4-phenylbutan-2-one to me |
....that is correct....thank you...solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Chordate
Hazard to Others
  
Posts: 108
Registered: 23-2-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Endimion17  | Why are English-speaking people almost always saying "sodium metal", "titanium metal", instead of sodium and titanium? I don't see anyone saying
"iron metal" when chemistry is discussed. It's iron, and it's obvious it's a metal.
Is the "_metal" addition required by BE/AE chemical nomenclature (it sure isn't required by the IUPAC standards), or is it a consequence of
proliferation of errors over the Web? I ask that because I've had more than a few brushes with pre-Internet English chemical literature, and such
linguistic constructs are very rare.
Also, the reagent bottles always say stuff like "CALCIUM", and beneath, written in small fonts, is "metallic" or "metal". If it's written in Latin, it
says "metallicum", but always as an additional info. The name of the substance isn't "CALCIUM METAL", it's "CALCIUM".
Since the Web has made a huge boom on the society, constructs such as "CADMIUM METAL" began replacing older, plain and simple "CADMIUM". Why is that?
It sound stupid, at least to me. Reminds me of errors that accumulate and soon become the "truth".
As it's something that is boosted by .com companies that sell compounds online. Sodium has become, from a notoriously difficult to obtain material
which I wanted to own since I saw its photos in elementary school, to a material easy to buy online, for tossing in lakes and toilet bowls by kids
whose chemistry knowledge is scarce. Everytime I hear "sodium metal", I instantly think of those kids.
I've grown up with bottles of "sodium", "natrium", "natrij", "sodio", etc., so yeah, I'm puzzled.
[Edited on 21-3-2012 by Endimion17] |
I think this primarily has to do with specificity. There are a lot of biochemists and biologists who also discuss sodium channels and sodium/potassium
pumps and clearly they are not talking about the metal. Halogens have an easy suffix to distinguish between their elemental and ionic states, and
those that were known to have exotic oxidation states before the formalization of modern oxidation state nomenclature still use common names like
"periodinane". Since metals are capable of attaining such a wide variety of oxidation states and still retaining ionic character and the names get
increasingly ridiculous, more and more people have moved over to the [element](oxidation state) state nomenclature.
But for sodium, calcium, and a few others, its just as easy to specify the ground state oxidation state by just saying "metal" and I think it has
spread because its both easy and scientists love to use precise jargon when they can.
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
I have 2 gallons of unopened HTH 10% sodium hypochlorite pool chlorinator. I bought it last summer, and stored it in the basement (cool and dark). How
much do you think it has degraded? Is it still useable?
|
|
|
Nathaniel
Harmless

Posts: 15
Registered: 5-4-2012
Member Is Offline
Mood: No Mood
|
|
Open it and carefully smell it - if it stinks like chlorine there's still some left 
This is just a quick test - you can pour some technical HCl on it (10ml +10ml for example)...if yellowish-green chlorine evolves the solution is
enough concentrated for most uses... Be careful when doing this (do it outside, because chlorine can be quite nasty).
It's hard to guess but if you want to know the specific concentration and have the chemicals and equipment you can titrate it as well (I once did it
with bleach): dissolve some iodide (1-2 grams KI) in water and add a few drops of sulfuric acid (more if it's not concentrated). Add an approximate
volume of your solution (about 15ml). The solution turns brown because of iodide. Titrate with thiosulfate solution until the colour fades-then add
some starch (turns blue) and continue titrating until it becomes almost colourless.
If you just need it for cleaning or something than testing it with HCl is enough 
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by Chordate  | I think this primarily has to do with specificity. There are a lot of biochemists and biologists who also discuss sodium channels and sodium/potassium
pumps and clearly they are not talking about the metal. Halogens have an easy suffix to distinguish between their elemental and ionic states, and
those that were known to have exotic oxidation states before the formalization of modern oxidation state nomenclature still use common names like
"periodinane". Since metals are capable of attaining such a wide variety of oxidation states and still retaining ionic character and the names get
increasingly ridiculous, more and more people have moved over to the [element](oxidation state) state nomenclature.
But for sodium, calcium, and a few others, its just as easy to specify the ground state oxidation state by just saying "metal" and I think it has
spread because its both easy and scientists love to use precise jargon when they can. |
Why being specific is some things are obvious? Biochemist obviously won't talk about metallic sodium.
What about "chlorine gas"? What's with the "gas"? It's obviously a gas. And why not "chlorine nonmetal"?
And bromine, iodine? You never hear "bromine liquid" or "iodine solid", although there's a huge and important difference between the elemental states
and the elements, a difference often used.
I don't know, dude. I've layed lots of arguments here to back up my hypothesis that this has something to do with n00b proliferation.
Quote: Originally posted by barley81  | | I have 2 gallons of unopened HTH 10% sodium hypochlorite pool chlorinator. I bought it last summer, and stored it in the basement (cool and dark). How
much do you think it has degraded? Is it still useable? |
It's fine.
[Edited on 6-4-2012 by Endimion17]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by barley81  | | I have 2 gallons of unopened HTH 10% sodium hypochlorite pool chlorinator. I bought it last summer, and stored it in the basement (cool and dark). How
much do you think it has degraded? Is it still useable? |
Usable for what? As a rough estimate I'd say it is likely to have lost 25% of its strength, i.e. it will function more like 7.5% sodium hypochlorite
and have a bit of sodium chlorate contamination as well.
The less you bet, the more you lose when you win.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Can H2S be used to destroy Chlorate contamination in Perchlorate?
Dann2
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Can NMP be safely distilled without a vacuum? If so, how about in a heating-mantle?
|
|
|
Mik229
Harmless

Posts: 4
Registered: 12-8-2010
Member Is Offline
Mood: No Mood
|
|
If nitrogen dioxide is bubbled through alkylhalide solution in aprotic polar solvent, is the only product nitrite or mixture of nitrite and
nitroalkanes?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Th other day i was making lead acetate and because it dries inn any surface quite well i had some on my spatula so i decided to remove it by dipping
it into a 50% solution of hydrogen peroxide....to my surprise it started fizzing and making foam and heating the beaker extremely hot....and
exothermic reaction but can't figure out the reaction .......
Lead(II) acetate (lead diacetate), Pb(CH3COO)2 +H2O2........2 PbO + GAA? not sure since this is the the way it was synthesized
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
What's up with this over-usage of little known (or custom, made up) abbreviations on this forum? They don't really make life easier.
Regarding the lead acetate thingy, I'd pay more attention to the 50% (holy shit) hydrogen peroxide decomposing and boiling. That's
why the solution went extremely hot.
If any lead(II) oxide was formed, it would be obvious by the precipitate.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Solo, some metals and their salts catalyze the exothermic decomposition of hydrogen peroxide. You should be more careful with 50% hydrogen peroxide.
As far as I know, concentrations of 60% and above can cause dangerous runaway decompositions (even explosions in closed vessels on a large scale).
Hydrogen peroxide with a metal catalyst can be used as a rocket fuel (see http://www.peroxidepropulsion.com/article/5).
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Does anybody know how and what the gel is made of in a gel mattress? I read it's made from plant and mineral oil but that is rather scant. It's
combined with polyurethane foam or bonded on top of it, I saw two samples the other day.
The elastomer reminds me of the texture of this toy, kind of sticky and very easily deformed. The gel is supposed to provide some cooling to the
mattress, as polyurethane foam can be hot to sleep on.
http://en.wikipedia.org/wiki/Wacky_WallWalker
[Edited on 23-4-2012 by Morgan]
|
|
|
Chemstudent
Harmless

Posts: 44
Registered: 3-3-2012
Location: Sunny Florida
Member Is Offline
Mood: Contemplative
|
|
I'm taking Organic Chemistry I this summer at my University. I have a good 3 weeks - 1 month before I take the class officially. I just completed Gen.
Chem I with a B, and had a good understanding of all the material. What is the best strategy for preparing my knowledge base for Organic Chem so that
I find everything already easy or familiar in 1 month? I've already studied and understood the little basics Alkanes/enes/ynes/cyclos & the
functional groups + conform/config/constitution of emil fischer projections. But thus far that is it. I figured just watch the Yale or other
University open courseware and take notes.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Quote: Originally posted by Chemstudent  | | What is the best strategy for preparing my knowledge base for Organic Chem so that I find everything already easy or familiar in 1 month? I've already
studied and understood the little basics Alkanes/enes/ynes/cyclos & the functional groups + conform/config/constitution of emil fischer
projections. But thus far that is it. I figured just watch the Yale or other University open courseware and take notes. |
Mastering the basics will significantly reduce the learning curve. Every O. Chem. professor is going to beat nomenclature and functional groups into
your head, so you were right to focus on it.
Next, start memorizing pKa values of common functional groups. This is a good table for that: http://mysite.science.uottawa.ca/aflynn/CHM2120_files/pKa_of... . Since proton transfers are the fastest reaction in organic chemistry, they are
always the first reaction in a mechanism. Understanding which groups will be protonated and deprotonated is critical to rationalizing reaction
mechanisms.
Review your thermodynamics and acid-base chemistry. These are also very important ideas in organic chemistry.
Start reading about NMR and Infrared spectroscopy. There are plenty of introductory resources available online and that is all you will need at this
point. Just learn (memorize) typical chemical shifts in NMR and absorption frequencies in IR, because that's another thing that introductory courses
will grill you on.
Good luck!
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Resonance is kind of tricky for some people. It's pretty intuitive once you know which groups are electronegative, etc. If you learn resonance
structures and hybrids before stepping in ontop of what you already know, you'll be completely set!(in my opinion)
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I recently found the need to prepare some DIB, ......one method in found in Molecule
Journal....
Molecules 2005, 10, 217-225
ISSN 1420-3049
http://www.mdpi.org
Application of [Hydroxy(tosyloxy)iodo]benzene in the Wittig- Ring Expansion Sequence for the Synthesis of β-Benzocyclo- alkenones from
α-Benzocycloalkenones
Michael W. Justik and Gerald F. Koser*
:.........................
Typical Synthesis of (Diacetoxyiodo)benzene
A solution of 32% (w/v) peracetic acid (100 mL, 421 mmol) in acetic acid was added dropwise with mechanical stirring to a cooled flask (15 oC)
containing iodobenzene (65.28 g, 320.0 mmol), over a period of 1 hour. The rate of addition was adjusted to keep the temperature of the reaction
mixture between 25-30 oC. Mechanical stirring was continued for 4 hours during which time a white precipitate separated. Water (100 mL) was added to
facilitate precipitation and to dilute any remaining oxidant. The solid was isolated by vacuum filtration, washed with water (2 x 100 mL) and ether
(150 mL), dried over P2O5 in a vacuum dessicator overnight and identified as (diacetoxyiodo)benzene; yield 94.86 g (92%); mp 160-161 °C (lit. [9] mp
159-161 °C).
.....it seems straight forward, any pitfalls i should be aware of...?....thanks...solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
| Pages:
1
..
15
16
17
18
19
..
30 |