| Pages:
1
..
15
16
17
18
19
..
25 |
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
All aluminum-silicon alloys present as a mixture of dentritic aluminum and flat silicon plates. The eutectic is something like 15%, so stuff below
has silicon as fine plates when the eutectic freezes around whatever aluminum crystallized from it. Above, fat silicon crystals form first, and
without additives, they are visible by eye on a cut surface (practical hypereutectic alloys, used for forgings and abrasion-resistant castings, have
grain refiners which make the crystals finer).
I've not isolated a crystal, but I gather they are generally shaped like graphite in gray cast iron; flat plates, oblate cross section, but with the
notable exception that silicon is an awful lot harder than graphite, so the alloys are a bit stronger than gray cast iron.
No intermetallics form at any point, at any rate of cooling, as far as I know.
How about something more mundane, like excess aluminum producing hydrogen which was ignited by an unusual concentration (near 1%?) of phosphide? Does
diphosphine usually ignite spontaneously at such concentrations? There is always a phosphide impurity, even in pure aluminum metal, it's just not
that much; however, it's plainly obvious in combination with acid.
Tim
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Tim -
Phosphine: it's a possiblity but how to explain that the phenomenon isn't observed when dissolving the actual aluminium powder in HCl? A bit of a
chemystery, this one...
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Idunno- phosphate in the CaSO4 or stuff?
Tim
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, remember this post of mine, Tim? That appeared to suggest reducing P<sub>2</sub>O<sub>5</sub> with Al is possible. And I
think that's correct: even an extremely stable oxide like Ta2O5 can be reduced with Al, mainly because it's a pentoxide...
But where would the phosphate come from? Phosphate deposits don't seem to coincide with Gypsum deposits at all (presumably because of their strongly
different solubilities). Most drywall must therefore be essentially phosphate free, IMHO...
Gangue accompanying the fluorite? Mine is made of clean off-grade lumps, no gangue in sight...
I guess the first step to solving the puzzle is to identify whether the gas in question is silane or phosphine... I might try that...
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
blogfast-If you can protect your reactive gas, then simply passing it through a heated glass tube at 400C or so should deposit a residue of red
phosphorus.
Fellow molecular manipulator
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chloric1
blogfast-If you can protect your reactive gas, then simply passing it through a heated glass tube at 400C or so should deposit a residue of red
phosphorus. |
Collecting the gas/hydrogen mixture and burning it in controlled conditions would be simpler: in the case of silane, silica (and possibly elemental
silicon) would be formed. Although vapour deposited silicon would be a nice thing to see...
[Edited on 5-10-2008 by blogfast25]
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
I have been promising to do this for months now, but I was just now able to perform Energy Dispersive X-ray Spectroscopy (EDAX) on some of my metal
samples prepared through thermite reactions.
For those of you who may not know,
EDAX works by firing a high energy electron beam at a sample. The high-energy incident electrons have a chance to eject one of the inner electrons
from an atom in the sample, thereby leaving a 'hole' behind in one of the atom's inner shells. An electron from one of the atom's outer shells
quickly falls down into this hole, in the process releasing a high-energy (X-ray) photon. By studying the spectrum of emitted X-rays we can tell what
elements make up a sample, and in what proportions. The majority of the readings will come from photons emitted at or near the surface of the sample,
however with a sufficiently high-energy electron beam one can penetrate a considerable distance into the sample (on the order of hundreds of
nanometers to microns deep).
I performed this analysis on small nuggets of "Silver" and "Titanium" metal which I produced via Ag2O and KClO3-boosted TiO2 thermite reactions.
The results are as follows:
Silver:
15 kV beam (large scan area, ~microns):
Weight percent: Carbon 7.29%, Magnesium 0.72%, Aluminum 0.68%, Silver 91.31%
Atomic percent: Carbon 40.25%, Magnesium 1.96%, Aluminum 1.68%, Silver 56.11%
20 kV beam (localized scan area, ~hundreds nm):
Weight percent: Carbon 11.96%, Magnesium 1.03%, Aluminum 0.74%, Silver 86.27%
Atomic percent: Carbon 53.38%, Magnesium 2.27%, Aluminum 1.47%, Silver 42.88%
Titanium:
20 kV beam (large scan area, ~microns):
Weight percent: Carbon 5.18%, Aluminum 24.78%, Titanium 70.04%
Atomic percent: Carbon 15.34%, Aluminum 32.66%, Titanium 52.00%
20 kV beam (localized scan area, ~hundreds nm):
Weight percent: Carbon 5.13%, Aluminum 23.50%, Titanium 71.37%
Atomic percent: Carbon 15.33%, Aluminum 31.23%, Titanium 53.44%
As you can see, the peaks for the nominal metal are very high, but they are not alone. There are metallic impurities in the nuggets, and apparently
some Carbon is in there too somewhere (possibly just residue on the surface it has picked up over time after I first made the metal and / or all the
handling I have done of it since then). The high presence of Aluminum is not surprising since Aluminum was present in the thermite reaction as the
reducing agent; finding that some of the Aluminum alloyed itself with the produced metal was expected, actually it was this ratio (Aluminum to Ti)
which I wanted to determine. The Magnesium in the Silver is also understandable since that thermite reaction did not really 'take off' after
ignition. Instead, once ignited, the Ag2O thermite tended to 'blow itself out', thus only a small area right around the Magnesium ribbon fuse
reacted.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hi Jeffrey,
This is very exciting (no pun intended) and somewhat unusual too.
I'm reasonably assuming that your samples of titanium are like mine and haven't been de-slagged. This is quite an important point because as you'll
recall I found a substantial part of the samples (typically a few g of material) to be insoluble in a excess of hot (near BP) 32 w% hydrochloric acid
(soaked for about 12 h). The residue typically weighed in at 10 - 15 w%. I always assumed that these were slag inclusions, so mainly annealed alumina
but never got a chance to verify this (fusing with alkali or NaHSO<sub>4</sub> should dissolve it I imagine).
And on the soluble part my titrometric analysis (Ti<sup>3+</sup> + Fe<sup>3+</sup> ---> Ti<sup>4+</sup> +
Fe<sup>2+</sup>, SCN<sup>-</sup> indicator) found roughly 80 w% of Ti. That would be broadly speaking in line with your
results, assuming I'm right about the slag inclusions.
The drawback of EDAX is of course that it doesn't tell you what molecular/ionic species the element is part of. Some aluminium must be present as
aluminium, but some may be present as alumina, as I suspect.
It's unfortunate that you don't have more ready access to EDAX because I feel the % of Al could be further suppressed by slagging excess TiO2 into the
thermite mix, as this would push the equilibrium TiO<sub>2</sub> + 4/3 Al <---> Ti + 2/3
Al<sub>2</sub>O<sub>3</sub> to the right.
I once estimated the equilibrium constant K of this reaction to be10<sup>3</sup> to 10<sup>4</sup> but that doesn't really fit
the high Al content. Still, the fact that we roughly use stoichiometric mixtures for this thermite and the fact that the enthalpy of reaction is quite
small (- 161 kJ/mol of reduced oxide IIRW, it greatly affects K, K being larger for more negative ΔG), would favour some of the Al not being used
to reduce the oxide.
The biggest headache though IMHO is the carbon content: 15 at%??? Blimey, who ordered that? I would have expected such a quantity to show up
during dissolution, wouldn't you? I never saw that...
What, in your opinion could be the source? Some aluminium powder (like German Black and some pyro grades) does contain quite a bit of carbon but as
far as I know mine doesn't...
Some titanox grades are surface treated (wetting agents, or silanes) and could contain some too...
If you get the chance to do some more EDAX, could I slip a sample (or two - lol) in there?
[Edited on 14-10-2008 by blogfast25]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
When I got an electron microscopy and x-ray spectrum of a sample, I was told C, H and O are always there as a result of surface contamination, even
freshly prepared surfaces. H and O come from adsorbed H2O, inevitable in the atmosphere, while C and H come from handling.
Tim
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
@blogfast-look more closely at Jeffy's data. I reread it myself for extra clarity. What became obvious to me was that the reading was off the
surface and the results with the 20kv beam verses the 15kv beam do in fact show LESS carbon which leads to surface contamination as the usual suspect.
Next time you have a chance at EDAX, I would try soaking samples in isopropanol, remove the sample and ignite remaining alcohol for sample surface,
vacuum dry sample, then seal in vaccum bag until testing can be commenced. Changing stochiometry to favor metal production will help minimize metallic
aluminum contamination. Many of the exotic metals we wish to produce are not alkali soluble so a bath in molten NaOH might help remove or loosen
alumina from sample. Alumina is quite brittle and some mechanical removal works as long as the metal is not so brittle. This is problematic with
chromium as I found it qute brittle and eaily broken despite hardness. This is where one only wants to really deal with larger pieces that are not
easily polverized.
[Edited on 10/14/2008 by chloric1]
Fellow molecular manipulator
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
The Titanium nugget came from a 530 gram thermite reaction composing of a mixture of TiO2, Al, KClO3, and CaF2 in a ratio of 100 : 75 : 50 : 40.
Ordinary, pure, Aluminum powder was used, not German Aluminum powder. After the reaction, the metal was removed from the rest of the slag (through
hammering) and was polished to get a shiny surface with a rotary grinder and sand paper.
EDAX will not tell you the chemical compounds making up the sample, just what elements are they. But if you already have a good idea of what your
sample is (for example, you know its some metal alloy, or you know its an oxide, ….) you can sometimes infer the ratios of the compounds you
suspect. In this case, however, the only elements which show up are ones which we would not ordinarily expect to bond together making some molecule /
compound, so I infer they are in their metallic state alloyed together.
It is surprising to me that no Oxygen showed up in the analysis (since there is no Oxygen peak). Had there been significant quantities of oxides (be
they Al2O3 or TiO2) in the metal then we would seem them. Apparently any oxides are only there in negligible quantities, drowned out by the metallic
Titanium and Aluminum.
The Carbon peak, although not unexpected from the presence of any surface contamination from handling, is fairly high. I must have found a dirty spot
on the sample and not the spot I had cleaned before hand.
I will have other opportunities to use the EDAX, but I cannot do it too often, only when my other projects (ones which legitimately require the use of
a scanning electron microscope) allow it. Next time I will make sure I clean the sample better.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
So, the high carbon reading could be explained away by sample contamination, until a better run can be made.
But the auspicious absence of O and N is also puzzling: you'd expect something made from an oxide and in the presence of air to be contaminated with
both. I have some process descriptions for Vanadium that require pumping vacuum in the thermite reactor and backfilling with argon, to avoid all O and
N. These, if I recall well, affect ductility of the metal...
Perhaps it would also be better to cut the nugget in half and polish one of the sides?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
O, N, B, C and more, all negatively impact the characteristics of titanium. Which is why it's so hard to make good stuff, and which is why chlorides
are usually used to make it.
It doesn't take much, on the order of parts per million, to affect the metal, but percents are barely detectable in the above method (what C is
present shows up as a scant peak). So maybe the O and N content really is pretty low, as far as the measurement goes, but may actually be rather high
as far as metal is concerned.
BTW, have you gotten around to smashing one of these nuggets? If they're so chock-full of stuff, they should be nice and brittle, something like
chromium, and shatter into a million bits.
Tim
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I have one chromium nugget about 1 cm and it is HARD. The metal from the thermite was brittle as shown by the smaller pieces. I wacked this nugget
with a 20 ounce hammer and it rolled away in defiance. So the small particles cool quicker and the 'junk" gets trapped and good crystaline structure
does not have a chance to form. The bigger ones stqay fluid long enough allow the "junk" to rise to the surface. Fluxing allows for bigger nuggets
to form.
Fellow molecular manipulator
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Tim:
I would say the thermite titanium is decidedly brittle, yes. But I don't really have a point of comparison (I do have some high purity Ti but I'll be
damned if I try and smash these samples up  ). ).
Also, the thermite titanium contains some non-metallic acid insoluble residue, not exactly conducive to high quality metal, I guess... This may well
be far more detrimental than any other impurity.
And ppm O and N wouldn't show up in Jeffrey's spectra, true. Not sure whether EDAX is suitable for such low level detection of these light elements?
Also, the choice of reducing TiCl4 with Mg has multiple motives, not just purity (but that plays an important part). A semi-continuous, relatively low
temperature process with relatively cheap and plant-integrated recovery of the reductant by electrolysis of the by-product MgCl2, is always an
attractive option. Chances are that even if the resulting metal would have required extensive refining (it doesn't), the Kroll process would still
have found a way into the market...
[Edited on 16-10-2008 by blogfast25]
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
I have not purposely tried to smash one of the Ti nuggets to pieces. However, during the polishing process, I do recall instances where some of the
smaller nuggets would crack and break apart due to all the stress being placed on them by me pushing them into the grinding wheel so hard and the
pressure of the pliers I was using to hold them. So they are somewhat brittle. They did, however, withstand the extraction process after the
thermite reaction, which involves a lot of hammering to break off the Al2O3 slag surrounding the Ti metal.
No, EDAX is not suitable to determine the presence of elements in such low concentrations as parts per million. Theoretically, it could detect them,
but their signals would get lost in the background noise (which is subtracted out at the end before quantification).
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I am attaching an article on iron oxide/aluminum thermite
While the oxide is not remotely exotic, it is synthesized by sol-gel methods leading to combustion velocities on the order of >800m/s. Hence why I believe this to count as exotic Hence why I believe this to count as exotic
Attachment: iron oxide thermite with very high velocity via sol-gel.pdf (1.4MB)
This file has been downloaded 1590 times
|
|
|
nitric
Harmless

Posts: 40
Registered: 18-8-2008
Member Is Offline
Mood: nitrous
|
|
has anyone done a thermite reaction involving Mg instead of Al, i heard somewhere that there more heat and shock sensitive
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
If you had bothered to read the thread, you would already know the answer.
|
|
|
Nixie
Hazard to Others
  
Posts: 490
Registered: 12-12-2006
Member Is Offline
Mood: ?
|
|
Cough... Kettle. Pot.
\"Good is a product of the ethical and spiritual artistry of individuals; it cannot be mass-produced.\" --Aldous Huxley
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I haven't had the opportunity to work some more on the MnCl2 + Mg reaction but I did run the first tests on the aluminothermic reduction of
niobium pentoxide, with interesting results so far.
About four 20 g reactions have been carried out with the base formulation (#1) being:
------------------------------------------------------------ mol
Nb<sub>2</sub>O<sub>5</sub> ...................................................................... 1
KClO<sub>3</sub> ................................................................... 0.05
Al ............................................................................. 3.4333...
CaF<sub>2</sub> .................................................................... 0.42
Right from the off, niobium metal was obtained, but unfortunately the small chunks were locked into the alumina matrix and complete separation of
metal and slag hadn't occurred.
Followed then some more attempts in which I increased firstly the flux level, then also the booster quantity (KClO<sub>3</sub> to 0.1,
then to 0.2 mol, adjusting also Al content of course).
The best result was obtained so far with KClO<sub>3</sub> = 0.1, where neat, shiny pieces of niobium were obtained. But even there,
metal/slag separation wasn't perfect.

Above a small lump of niobium metal (about 3 g), broken in two pieces. Externally, slag adheres very strongly to it. The metal is the typical
'silvery, gray' you find in so many descriptions of transition metals.
This thermite is unique in one respect from the many others I've successfully pulled off: the melting point of the metal (2,477 C) is actually higher
than the melting point of the slag (2,054 C), so on post-reaction cooling it's the metal that solidifies out first, with the still molten slag
'dripping off' the metal. The amount of time the metal has to separate out is also quite small: in adiabatic conditions the expected
end-temperature of the reaction is about 2,700 - 2,800 C, so on (natural) cooling the solidifying point of Nb will be reached quickly. I wonder also
if solidifying micro droplets of niobium in the melt will not increase the apparent viscosity of the melt. The 'ideal' of a perfectly flat slag/metal
puddle with all the metal neatly collected at the bottom of the crucible, may therefore be difficult to achieve in the case of the niobium thermite.
While chlorate heat-boosting is paramount to success, it hasn't got the overwhelming effect that it has in lower energy thermites (such as titanium)
because the actual reduction reaction is already so energetic (ΔH = - 962 kJ/mol of pentoxide). The booster reaction has a ΔH = -1,255
kJ/mol of chlorate. As a result, theoretically the booster reaction increases the end-temperature of the mix only by about 55 C per 0.05 mol of
KClO<sub>3</sub> added.
As a consequence I believe that if too low end-temperature really is the problem here, a combination of preheating the mix and using chlorate
heat-boosting is probably the solution. Larger size batches may also improve things, as they retain heat a little longer.
So the next attempt will be aimed at carrying out the reaction al forno.
[Edited on 9-12-2008 by blogfast25]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Titanium by Aluminothermy
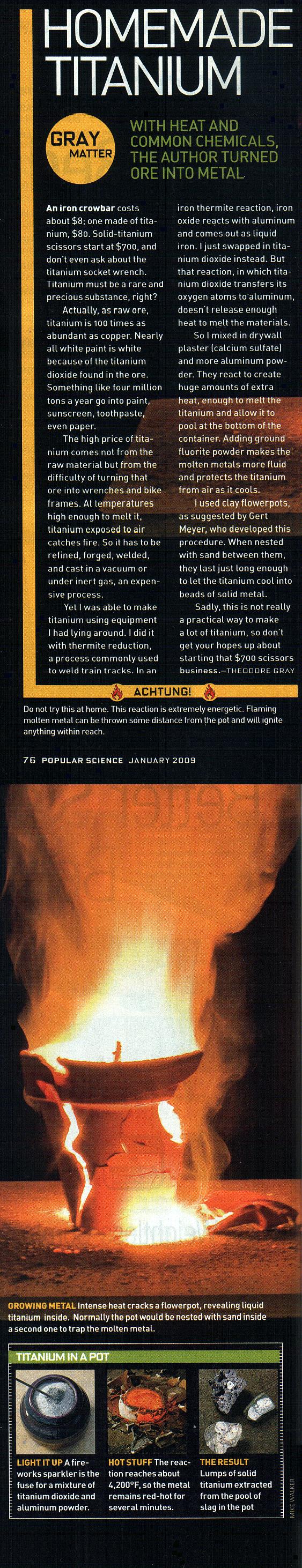
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Well, well, someone has already found the printed issue of Popular Science here. The "Gert Meyer" referred to is me (although Theo misspelled my name
slightly, it's actually Meyers).
Following a tip-off by 12AX7, who is also forum master at ABYMC (where I first reported calcium sulphate boosted TiO<sub>2</sub> , Theodore Gray and me got in touch about him writing an article about homemade
titanium. Months of waiting, guiding and much toing and froing later, Theo reproduced my results (described in great detail here) on a grander scale and took some awesome pictures. He wrote the article based on his experience. Apparently it
made it to the cover, with photo! , Theodore Gray and me got in touch about him writing an article about homemade
titanium. Months of waiting, guiding and much toing and froing later, Theo reproduced my results (described in great detail here) on a grander scale and took some awesome pictures. He wrote the article based on his experience. Apparently it
made it to the cover, with photo!
Personally I hadn't seen the printed issue yet (being in Europe), but he's sending me one...
[Edited on 16-12-2008 by blogfast25]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
From a now deeply buried post 2 years ago , a very useful excerpt on techniques
for welding and repair of large broken structural and mechanical parts in the field ,
first published in 1910 , worth a look if you missed it the first time - http://ifile.it/o4pd7rm
.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Toxic site contamination
Mercuric oxide should be a vigorous oxidant in a thermite-type reaction. Only useful if you really wanted to contaminate a site, though.
|
|
|
| Pages:
1
..
15
16
17
18
19
..
25 |