| Pages:
1
..
13
14
15
16
17
..
20 |
gboneu
Harmless

Posts: 30
Registered: 12-5-2015
Member Is Offline
Mood: No Mood
|
|
When i was using at som TCCA (Trichloroisocyanuric acid) which is used in pools as Chlorine tablets, When i used Ethanol with this in over 5 min. A
student reaction occurred and my lab was smelling Acetaldehyde .xD So i believe that controlling the Temperature might me the trick and you can use
it, Though you mey need a catalyst because a little of anhydrous oxalic acid was present and the ethanol wal 99,5% (Anhydride) You can try ir out with
95% ethanol, and no Oxalic acid.
NOTE: I didn't heat the reaction, it just went by it's self
[Edited on 12-5-2015 by gboneu]
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Hi, today I've got an idea on how to make acetaldehyde, using copper catalyst. I'll add my drawing below... I think I'll try it, but I need to buy
aquarium air pump and hose barb to ground glass joint adapter (I don't know where I'd be able to get one though - size 29/32). I'd be happy for you to
let me know what do you think about this setup - do you think it would work?
Thanks 
[Edited on 24-5-2015 by xfusion44]
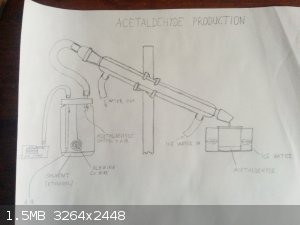
[Edited on 24-5-2015 by xfusion44]
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by xfusion44  | Hi, today I've got an idea on how to make acetaldehyde, using copper catalyst. I'll add my drawing below... I think I'll try it, but I need to buy
aquarium air pump and hose barb to ground glass joint adapter (I don't know where I'd be able to get one though - size 29/32). I'd be happy for you to
let me know what do you think about this setup - do you think it would work?
Thanks 
[Edited on 24-5-2015 by xfusion44]
[Edited on 24-5-2015 by xfusion44] |
That looks like a pretty good method as for affordability. Keep in mind
however that you will also end up with a lot of Acetone in your product, so it will likely have to be purified extensively. You shouldn't need a
ground glass adapter for the tube to condenser, use a cork fitted with thevtube. And push that into the socket. Nice
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Beautiful sketch!
What's to prevent a fire (explosion) in that jar?
As the previous commenter said you will have by-products like acetone and unreacted ethanol in the condensate. How will you separate them from the
acetaldehyde?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Thanks to both of you 
@hawkguy
I'm a little bit confused now. I thought that ethanol would produce acetaldehyde, and acetone something else? Do they both produce acetaldehyde?
Otherwise, I don't think that there would be a lot of acetone in acetaldehyde, because I would not boil it during this process, I'd just leave it to
evaporate slowly and efficiently - am I wrong?
@Magpie
I also thought about possible explosions, but haven't yet come up with idea about how to minimize the risk. Probably one would just need to accept the
risk when performing this - safety equipment would be necessary here.
BR, xfusion
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
see: http://www.sciencemadness.org/talk/viewthread.php?tid=55&...
(post of organikum)
Also, see jimmymajesty's fine work, this thread
Quote: Originally posted by xfusion44  |
Probably one would just need to accept the risk when performing this - safety equipment would be necessary here.
|
I'll be standing at the door with a face shield on.
No, I would not do this experiment unless first convinced I would not have an explosion.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@Magpie
Thanks!
Maybe you could just press the lid on top of the jar and seal the gap with PTFE - this should act as a safety valve, so that in case of explosion it
would just blow the lid off of the jar.
[Edited on 24-5-2015 by xfusion44]
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
If you want to try something quick and dirty, I would suggest this configuration over doing it in a jar.

The wad of copper wire on the left is intended to function as a flame arrester. It should stay cool during normal operation in order to provide this
function. The copper wire on the right is a wad of very fine wire (to provide as much surface area as possible). These wads of wire are pushed down
into the glass tube, which is then bent appropriately with a propane torch. The left side goes into a rubber stopper in an Erlenmeyer flask that
contains ethanol. The ethanol is heated slightly in a warm water bath to increase its volatility. In the same stopper, a glass tube is pushed under
the ethanol surface, and air is slowly bubbled through. This forms a fuel/air mixture. During operation, the copper on the right can be
heated gradually with a propane torch. If the fuel/air mix is correct, the copper will glow even when the torch is removed. This is kind of fun to
look at in the dark.
Just because it is safer than doing it in a large jar doesn't mean it is completely safe. Certainly use a face shield. Leather welding gloves are a
good idea if it is necessary to adjust things during operation. Even better, a Plexiglas blast shield can be added for extra safety.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@WGTR
Thanks for the idea 
Does it work well? Where does the right side of the tube go? To the condenser? How about efficiency?
BR, xfusion
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
+1 for question about effectiveness. Catalyst has a limited activity, so there's also a question about whether ethanol should be boiling, or maybe
some inter carrier or air is passed through it without ethanol boiling. I think something like 70°C and air will do the job. Because boiling ethanol
is hard to control, and higher amount of oxygen will lead to oxidation into CO2.
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by xfusion44  | Thanks to both of you 
@hawkguy
I'm a little bit confused now. I thought that ethanol would produce acetaldehyde, and acetone something else? Do they both produce acetaldehyde?
Otherwise, I don't think that there would be a lot of acetone in acetaldehyde, because I would not boil it during this process, I'd just leave it to
evaporate slowly and efficiently - am I wrong?
@Magpie
I also thought about possible explosions, but haven't yet come up with idea about how to minimize the risk. Probably one would just need to accept the
risk when performing this - safety equipment would be necessary here.
BR, xfusion |
Sorry, I thought you were doing the reaction with Acetone. Thats the way I've done it before, because of its boiling point, and I haven't had any
explosions with it like I have with Methanol or Ethanol..
|
|
|
Nicodem
|
Thread Split
25-5-2015 at 07:02 |
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by xfusion44  | @WGTR
Thanks for the idea 
Does it work well? Where does the right side of the tube go? To the condenser? How about efficiency?
BR, xfusion |
No, it does not work very well. Yes, the right side of the tube goes to a condensor. The efficiency (or my patience) was poor enough that I couldn't
get quantitative amounts of acetaldehyde from it. At the same time, it will be more effective than a copper spiral in a jar, and much safer. It will
also give an idea of how air flow and fuel mixtures affect its general operation. And yes, one can smell some pretty pungent products coming out the
other end.
Now, to explain the previous a little better: Temperature control was very difficult with this setup. Yes, the initial heating was accomplished with
a torch. However, the thin wire of the catalyst offered poor heat transfer, so localized overheating would occur during operation. The bulk of the
wire catalyst was not used evenly. Most of the reaction would occur at one localized area in the bulk wire, usually at the leading edge. The
self-sustainability of the reaction was determined by the air-fuel ratio. This in turn was determined by the alcohol temperature and the
effectiveness of the air bubbler. As byko3y mentions, it is easy to overoxidize the product.
The air volume was very low. I would estimate about 1 cm^3 per second, but that is probably too fast for this particular setup. When one considers
the low air flow involved and that alcohol vapor is a only small fraction of this, it's not surprising that one will wait a very long time for that
first mL of acetaldehyde. In "Catalysis in Organic Chemistry" (Sabatier) pg. 654, a column full of copper catalyst 25-30mm in diameter, 1 m in length
was used to prepare 500g of acetalydehyde per day. That gives an idea of how slow this process can be.
I gave up on the idea of oxidative dehydrogenation, and instead focused on direct dehydrogenation. The latter process requires the continual addition
of heat to continue, so it is less likely to overheat. It also avoids the use of oxygen, so the products are free of acetic acid, water, etc. I
detailed the construction of this idea here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=55...
Also, JimmyMajesty's setup is quite impressive, and works on similar princicples. He has done well with the output condensor design, which is
something I have yet to do properly. The acetaldehyde product will contain a large amount of unconverted ethanol. For this reason it is always
necessary to seperate out the ethanol from the products, and send it back to the boiler. The condensor will need to be able to separate out this
alcohol from the acetaldehyde, and also to efficiently condense out the aldehyde. This is easier said that done, as one can see when reading back
through JM's posts in this thread.
I would strongly advise reading through Sabatier's book on catalysis. It was such an instructive and interesting book, that I almost read it
cover-to-cover in one sitting. It is available for free all over the internet. It will help you more than I possibly could in a thread.
That is a nice drawing, by the way.
Quote: Originally posted by byko3y  | | +1 for question about effectiveness. Catalyst has a limited activity, so there's also a question about whether ethanol should be boiling, or maybe
some inter carrier or air is passed through it without ethanol boiling. I think something like 70°C and air will do the job. Because boiling ethanol
is hard to control, and higher amount of oxygen will lead to oxidation into CO2. |
The temperature I was using was about 40-60C. It has to be adjusted to change the air-fuel ratio. I would generally get the alcohol concentration as
high as I could, while it would still allow a self-sustaining reaction in the catalyst.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@Hawkguy
That's exactly why I asked you about using acetone. If the product is the same, it's really better to use acetone because of its lower BP. But when
I'm using acetone instead of ethanol, the smell is more pungent and I think, a little bit different, or is it just me?
@WGTR
Thanks!
Looks like you have a lot of experience with acetaldehyde production  Well, you
could use copper foil in the jar or even copper wool, but as we already know, it's not very safe to do it with the jar method Well, you
could use copper foil in the jar or even copper wool, but as we already know, it's not very safe to do it with the jar method 
BR, xfusion
|
|
|
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by xfusion44  | Thanks!
Looks like you have a lot of experience with acetaldehyde production  Well, you
could use copper foil in the jar or even copper wool, but as we already know, it's not very safe to do it with the jar method Well, you
could use copper foil in the jar or even copper wool, but as we already know, it's not very safe to do it with the jar method 
BR, xfusion |
You're welcome! There's more than one way to do things like this. I think that JimmyMajesty's setup has been the most practical overall, as he has
made the most here using direct dehydrogenation (as far as I know).
Certainly, as you try things out, post pictures and ask questions if needed. I enjoy seeing people's practical setups on this topic.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@WGTR
Thanks! When I try this, hopefully I'll remember to take some pictures 
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Hey if Aldehyde/ Ketones can be collected by a destructive distillation of a corresponding Calcium salt, can Acetaldehyde be produced from Calcium
Acetate?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
No, you don't get acetaldehyde, you get acetone. http://www.sciencemadness.org/talk/viewthread.php?tid=16216 It was suggested earlier in this thread, but calcium formate is required as well and
yields are pathetically low. http://www.sciencemadness.org/talk/viewthread.php?tid=55&...
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Rats I thought I found an easy shortcut. Well, back to the Dichromate style oxidation then.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2836
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
From Advanced Organic Chemistry, Fieser & Fieser, (c) 1961, found at a used book sale (^,^):
12.6 - Tosylate method - Kornblum found (1959) that primary saturated alcohols are converted into aldehydes in 60-85% yield by
oxidation of the tosylates with dimethyl sulfoxide (DMSO) in presence of sodium bicarbonate at 150C. Benzylic tosylates are oxidized smoothly at 100C.
The required esters can be produced from the halides; for example, by reaction of n-octyl iodide dissolved in acetonitrile with silver tosylate.
Ethyl halides might be more reactive than octyl, so the iodide might not be necessary... also if you have tosyl chloride or even [m]ethanesulfonyl
chloride you're probably set. Ethyl tosylate can also be prepared by the reaction of ethyl orthoformate with toluenesulfonic acid and fractional
distillation.
https://www.erowid.org/archive/rhodium/chemistry/sulfonic.es...
Silver tosylate can be prepared by salt metathesis from silver nitrate and sodium tosylate; AgOTs precipitates. See:
http://onlinelibrary.wiley.com/doi/10.1002/047084289X.rs030/...
Be aware: the autoignition temperature of acetaldehyde is 185 C. Don't blow yourself up. But ethyl tosylate is more reactive than
longer alkyls thus shouldn't have to get so hot.
[Edited on 2-8-2015 by clearly_not_atara]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Without the googly part, that's
https://www.ideals.illinois.edu/bitstream/handle/2142/4460/e...
There is also the earlier
https://www.ideals.illinois.edu/bitstream/handle/2142/4543/e...
Related paperwork using copper turnings, etc. (formaldehyde is also made from methanol)
Catalytic Partial Oxidation of Alcohols in the Vapor Phase—III
W. Lawrence Faith, D. B. Keyes
Ind. Eng. Chem., 1931, 23 (11), pp 1250–1253
DOI: 10.1021/ie50263a014
Catalytic Partial Oxidation of Alcohols in the Vapor Phase.IV
W. Lawrence Faith, P. E. Peters, D. B. Keyes
Ind. Eng. Chem., 1932, 24 (8), pp 924–926
DOI: 10.1021/ie50272a022
Attachment: ie50263a014.pdf (145kB)
This file has been downloaded 1298 times
Attachment: ie50272a022.pdf (161kB)
This file has been downloaded 850 times
[Edited on 15-11-2015 by S.C. Wack]
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Hi im working on a pentaerythritol synthesis and i am looking for information if you can calculate the % of acetaldehyde in a solution by using the
sodium sulfide method as you would to calculate a formeldehyde solution.
I have googled for info but havent had much luck finding any info online.
Thanks nux.
[Edited on 9-12-2015 by nux vomica]
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by nux vomica  | Hi im working on a pentaerythritol synthesis and i am looking for information if you can calculate the % of acetaldehyde in a solution by using the
sodium sulfide method as you would to calculate a formeldehyde solution.
I have googled for info but havent had much luck finding any info online.
Thanks nux
[Edited on 9-12-2015 by nux vomica] |
What would the Acetaldehyde be dissolved in... Ethanol?
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Yes its a mix of unreacted ethanol and acetaldehyde, I collect the distillate that comes off below 60° c but it would be better to know the % of
acetaldehyde in the solution so I can calculate how much to collect , and to check purity of the final product.
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by nux vomica  | | Yes its a mix of unreacted ethanol and acetaldehyde, I collect the distillate that comes off below 60° c but it would be better to know the % of
acetaldehyde in the solution so I can calculate how much to collect , and to check purity of the final product. |
Try making paraldehyde and measuring it that way
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hawkguy  | Quote: Originally posted by nux vomica  | | Yes its a mix of unreacted ethanol and acetaldehyde, I collect the distillate that comes off below 60° c but it would be better to know the % of
acetaldehyde in the solution so I can calculate how much to collect , and to check purity of the final product. |
Try making paraldehyde and measuring it that way |
I will have to look into that method, as I already use the sodium sulfide method with my formaldehyde solutions I was hoping it wold work with
acetaldehyde as well.
|
|
|
| Pages:
1
..
13
14
15
16
17
..
20 |