| Pages:
1
..
12
13
14
15 |
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
https://photos.app.goo.gl/16SA1f7RDucAvtGh6
hey, it`s pretty.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I have some of the green carbonate complex of cobalt III drying on filter paper, it's a wild color to see from cobalt
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
| Quote: |
Edit by Texium: Changed title because the "one true thread" will likely never reappear, unfortunately.
[Edited on 4-11-2023 by Texium] |
THAT'S NOT TRUE!! tHAT'S IMPOSSIBLE!!!
anyway 
There's a season of Trailer Park Boys which opens with Ricky collecting garbage from the park and trying to sell it back to the community. He offers
some beer cans to his quasigirlfiend Lucy, saying that they would look great decorating the kitchen. "No, that's cool, thanks, if I need any I'll come
get one," she says.
I mention this because I visited a weirdo friend of mine recently. With students out of the dorms, the dumpsters have been rich pickin's, and he
proudly showed me his haul. I expected something like kitchen items, books, a television, but no: he had an impressive pile of aluminum cans. He
explained to me that an even weirder friend of his used them as disposable pipes to smoke drugs off of before work, and thought he could use a few.
Okay, I said, but they've got paint and plastic on them, do you really want to encourage him breathing those fumes? Can't you just show him how to use
a carrot or an apple?
He insisted that there was no plastic coating on soda cans. I long ago learned that he doesn't regard any of the internet as a legitimate source,
believing that it has been irreparably corrupted by Vladimir Putin himself. It was show-me-not-tell-me time.

I scrubbed the outer layer of paint off of the lower half of a can of Diet Coke and suspended it from the tab in a beaker. Then I poured a moderate
sodium hydroxide solution around it, and left it to fizz overnight.

The next morning, I had a half aluminum can, half plastic bag, still containing the Coke.
When I showed it to my friend, I definitely convinced him of something. I'm not sure of what, precisely, but he clearly felt like he had been lied to
his whole life, about the nature of soda cans. He wants me to go on Christiane Amanpour and alert the general public.
And that's my adventure in the intersection of mad science, harm reduction, and the Trailer Park Boys IRL.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
| Quote: | Edit by Texium: Changed title because the "one true thread" will likely never reappear, unfortunately.
[Edited on 4-11-2023 by Texium] |
Can you explain?
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
The original thread was lost to the aether in some kind of tech glitch.
It is probably retrievable in one of the site backups, but practically, the information it contained is lost.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
day before yesterday, i massacred four innocent 1964 US 25 cent pieces in hot nitric acid. after two recrystallizations, i`ve got most of the blue
from the copper out of my AgNO3, and a bunch of black stains on my fingers. phone broke at the moment so no pics 
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I got my hands on some iodates/periodates and have been running through some of the experiments on woelen's site.
One thing I found was that the sulfur/periodate mixture is slightly shock sensitive; when wrapped in aluminum foil and tapped with a hammer there was a distinct smell of sulfur
dioxide, small points of purple-brown discoloration on the foil appeared, and there were even a few sparks. When I really went Hephaestus on it, I was
eventually able to get a hearty snap. The reaction didn't seem to propagate much; there was plenty of the mixture left to burn afterwards.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I've been trying to get the silver oxide oxidation of vanillin to work for ethylvanillin, salicaldehyde, and other related aldehydes, with limited
success. Some of these acids really don't like to recrystallize.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Always lable beakers
Doing some cleanup and the permanent marker lableing a wash beaker had wiped off. No biggie, I put the 3 times refined silver nitrate in the cupboard
last night and this was only rinse water. Ill just add my current rinse to it(cu+/hcl/k+/h2o2) and neturalize the whole lot later.
Son of a ;>/"& mother ahhhhhhh.
I run to my shelf, pull out my jar from last night, and discover its lable had also wiped off.
"You can't do that" - challenge accepted
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I'd never done a vacuum distillation before so I tried cleaning up a small amount of benzaldehyde that was languishing in the organics bin, turning
orange and growing crystals. Everything went better than expected! Nice clear and colorless liquid, with ~65% mass recovery from starting material I'm
pretty sure is at least 15% benzoic acid. The only problem was that it took a while for the vapor front to make its way through the claisen adapter,
and some moisture condensed in the receiving flask.... the last I fixed by putting it in the freezer and pipetting off the ice lumps once everything
was frosty 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 491
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
Dioxane, first time.

Oh, the joy.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
Phosphorous pentoxide + water = massively exothermic
Disposing of some P4O10
I slowly added it to running tap water to flush it away as dilute phosphoric acid,
which I believe is OK for the local waste water treatment plant.
I was surprised by how exothermic the reaction is
(more than conc. sulphuric acid or NaOH or KOH)
So much more dangerous than I'd expected,
based only on previous use in a diy dessicator.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I had my students try a new lab today- bromination of stilbene. It worked well, although it's tough to recrystallize the product (its solubility
doesn't change much with temperature, it seems).
Previous years had made 1,3-diphenylpropenone and a methoxyphenyl analgoue, so I figured, why not brominate these? The first one worked well, but the
second just gave oily crap. No idea why. Oh, well.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I've got a paper here (https://tesla.pmf.ni.ac.rs/lgc/articles/40-1992.pdf ) that discusses the synthesis and thermal decomposition of Cu(urea)4Cl2, made by reacting
copper(II) chloride with urea in a 1:2 ratio. Elemental analysis said it was 16.9% copper, consistent with the 1:4 adduct.
I reacted copper(II) chloride with urea in a 1:4 ratio under very similar conditions. My product is 24.6% copper, consistent with Cu(urea)2Cl2.
Grrrrr.......
[Edited on 22-12-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
Today after a long time I go back to the lab for making a printed circuit board. Lately I've been busy with other stuff and I'm almost not doing
chemistry anymore, but printed circuits are one of the applied chemistry that I still do from time to time, the other being analog photography.
However this time I found my photoresist developer, a sodium hydroxide and metasilicate solution, had crystallized. Likely due to the low temperatures
in the lab.
So I'm sitting here, watching crystals slowly dissolve and remembering when I was dreaming of doing complex reactions that I never got around to
trying, or failed.
Oh, well, I still have the lab, reagents and apparatus, so maybe someday I'll get back...

|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
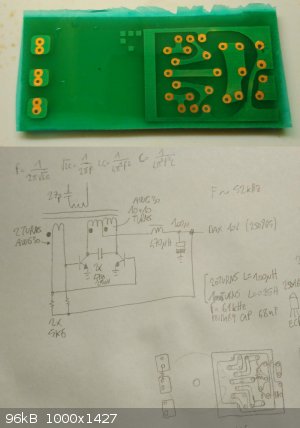
The resulting printed circuit
|
|
|
charley1957
Hazard to Others
  
Posts: 173
Registered: 18-2-2012
Location: Texas
Member Is Offline
Mood: Winter’s winding down. Yay!!
|
|
Good looking board, traces nice and sharp, crisp edges, good solder mask. I like it! Is that some kind of oscillator?
You can’t claim you drank all day if you didn’t start early in the morning.
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
It is an oscillator. More precisely, a Baxandall oscillator [1]. I'm using it to produce a high voltage sine wave to drive the neon tubes I'm making
(I've been learning the very basics of glassblowing). Here's the printed circuit with all components soldered and working

[1] Baxandall, P.J. "Transistor sine-wave LC oscillators: Some general considerations and new developments"
https://web.archive.org/web/20211222202712/http://www.sophia...
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Ran out of sodium sulfate and seen i was low on magnesium carbonate as well(mold release). So 2 birds 1 stone.
Weight out 1052g of MgSO4*5h20 and 530g of Na2CO3, dissolved each in a minimal amount of hot water, then mixed the two in a large bucket and is
currently settling. While I wait im dissolveing
293g of NaCl in water. Will decant the liquid into the salt bucket and apply a fan. Should have some nice aluminum flux in a few days. Great for
casting, prevents O2 absorption in the melt and decreases pouring viscosity.
[Edited on 1-6-2024 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
Measured density of mercury in my new 10ml pycnometer....
0.33% more dense than standard values.
Close, but not satisfied yet.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Been making a lot of pcb lately and ran out of etching solution.
MG Chemicals - 415-1L is $52 for 400g of FeCl3 (40% solution) in some water at the local supply house.
No no no.
400÷162.2 = 2.47mols * 55.845Fe =137.74g of iron.
150g of steel wool + h2o + hcl solution
Filter, top off to a nice orange with h2o2, and condence
[Edited on 26-9-2024 by Rainwater]

"You can't do that" - challenge accepted
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
What a time to do Chemistry.
Toxins, Organic Chemistry, Terpenes.
...
I mean, we could build a simple robot and not risk dying in the process.
I was working on this for a long time.
...
Toxins, Organic Chemistry, Terpenes. ... also RadioActivity and much more
This is just a random list. I listed Toxins because we are getting closer to safe handling of various toxins by using a simple Robot.
...
Just Random... also I could say Incomplete Thoughts.
Edit:
I have also been thinking for example about dry distillation of Printed Circuit Board.
I had more ideas also...
...
[Edited on 30-9-2024 by Random]
|
|
|
Sulaiman
International Hazard
    
Posts: 3779
Registered: 8-2-2015
Member Is Offline
|
|
out of
curiosity, what products are you aiming for?
I've watched many YT gold recovery videos,
a common practice is pyrolysis, with little care for the emissions.
So if you can devise something that captures emissions which is cheap and easy to construct that in itself would be a benefit.
If a part of the emissions can be used to assist with pyrolysis that may be a further benefit.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
dettoo456
Hazard to Others
  
Posts: 255
Registered: 12-9-2021
Member Is Offline
|
|
Earlier today I was attempting to neutralize a bucket full of ash/charcoal mixed in water with some old H2SO4 drain cleaner I had laying around (was
trying to convert all of available Ca in sol to Gypsum for some general soil amending in my backyard). Anyways, the minute I added around 50ml of the
conc drain cleaner, a large amount of bubbles formed as expected, but there was also an alarmingly strong smell of H2S as well that lingered.
Has anyone noticed this before? I’m assuming the sulfuric acid directly acted on the charcoal in the bucket although I didn’t think it’d
necessarily be possible, seeing as carbon on its own usually isn’t that reactive (especially when wet).
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 491
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
I think it’s more likely that there was some sulfide present. Sulfates, when heated with carbon, are reduced to sulfides.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
| Pages:
1
..
12
13
14
15 |