| Pages:
1
..
12
13
14 |
nleslie321
Hazard to Self
 
Posts: 62
Registered: 7-11-2017
Location: krakatoa
Member Is Offline
Mood: just right
|
|
yeah i am looking at things to use ketene for also. So far ive done acetic anhydride from GAA and propionic anhydride from propionic acid.
|
|
|
NZniceguy
Harmless

Posts: 48
Registered: 16-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by nleslie321  | | yeah i am looking at things to use ketene for also. So far ive done acetic anhydride from GAA and propionic anhydride from propionic acid.
|
Does ketene bubbled through propionic acid give prop. anhydride? Or do you use mek instead of acetone?
|
|
|
Keras
International Hazard
    
Posts: 1027
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Speaking of.
One of the classical high-school experience about organic chemistry is the copper catalysed oxidation of ethanol to ethanal. Over a beaker containing
warmed ethanol is placed a red-hot copper ribbon. Immediately, an "apple-like" scent is smelt, and the copper ribbon stays red-hot because the heat of
the oxidation is enough to make up for thermal losses.
So question: if acetone is used instead of ethanol, does one get:
1. Ketene?
2. water and carbon dioxide?
3. Nothing, because acetone cannot be oxidised this way?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2836
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Ketene is not an oxidation product; it is a thermal degradation product. If ketene were produced, though, the only thing it would be useful for is
suicide.
|
|
|
Keras
International Hazard
    
Posts: 1027
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | | Ketene is not an oxidation product; it is a thermal degradation product. If ketene were produced, though, the only thing it would be useful for is
suicide. |
I don't dispute this, and I have absolutely no intention to do it. I was just curious. Is the pyrolysis of acetone into ketene exothermic enough that
a red-hot copper ribbon stays hot even though no external heat source is present? I guess this would go somewhat against the setup of the ketene lamp,
where the electrical current is to be switched on at all times.
[Edited on 29-6-2019 by Keras]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
The copper catalyses
the formation of ketene (ethenone) and methane.
(CH3)2C=O → H2 C=C=O + CH4
The ketene and methane are oxidised (and presumably some acetone is directly oxidised as well), providing the necessary heat to keep the copper
glowing.
ketene is formed, but oxidized quickly after. if you hadn't oxygen presence the copper wouldn't heat up and keep glowing, stopping the formation of
ketene and it's oxidation.
the formation of ketene is endothermic, it's oxidation exothermic, that's why copper, in acetone vapours and oxygen keeps glowing, but to make ketene
you have to heat the wire
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Keras
International Hazard
    
Posts: 1027
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Okay, so that means you end up with carbon dioxide and water?
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Does ketene bubbled through propionic acid give prop. anhydride? Or do you use mek instead of acetone?[/rquote]
I've wondered about this myself.whats the answer? Does methylketene come into play here or will one get ketene and ethane? Or 2moles of methane?
Probably not on the double methane. I'd guess methylketene and methane.
[Edited on 5-7-2019 by draculic acid69]
|
|
|
nleslie321
Hazard to Self
 
Posts: 62
Registered: 7-11-2017
Location: krakatoa
Member Is Offline
Mood: just right
|
|
Yes ketene bubbled through propionic acid gives propionic anyhydride and you can also use methyl ethyl ketone to make methyl ketene and bubble through
GAA to get a mix of acetic and propionic anhydrides. ive done both methods and have both in my lab.
|
|
|
Waffles SS
Fighter
    
Posts: 1000
Registered: 7-12-2009
Member Is Offline
|
|
When Ketene is scrubbed with fatty acids higher than acetic acid such as propionic acid or butyric acid and the like, the mixed anhydride of acetic
acid or of a homologous acid and the higher fatty acid is formed.The mixed anhydride on heating rearranges and the separate anhydrides of both acids
form.By distillation these can be separated.(same will be happen by reaction of methyl ketene with GAA)
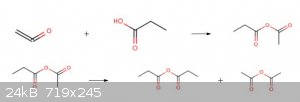
[Edited on 31-7-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by nleslie321  | | Yes ketene bubbled through propionic acid gives propionic anyhydride and you can also use methyl ethyl ketone to make methyl ketene and bubble through
GAA to get a mix of acetic and propionic anhydrides. ive done both methods and have both in my lab. |
Wow finally I can cross that question out on my list of questions to find out I wrote down about 12 years ago.it also sounds like why bother with
acetone when mek can do two anhydrides with one ketone.this is awesome news.only thing is what ratios are the acetic/propionic formed? equal amounts
or 90%10% ?
|
|
|
nleslie321
Hazard to Self
 
Posts: 62
Registered: 7-11-2017
Location: krakatoa
Member Is Offline
Mood: just right
|
|
From memory i think it was a little more propionic anhydride than acetic anhydride via mek pyrolisis. Its a very simple reaction to do if you want to
try it.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
If and when I try this rxn I'll be using mek.
|
|
|
| Pages:
1
..
12
13
14 |