| Pages:
1
..
12
13
14
15
16
..
38 |
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yep. I'm lucky I didnt leave any other condensers hooked up to the water recirculator. I'm pissed off enough as it is.
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Oy ve! I understand that not all people can have a lab indoors, but leaving expensive stirplates and valuable precicion glassware in a cold damp
environment where freezing can occur is not a good idea by any definition...
This unfortunate Friedrichs condenser is a perfect example of gear that should have been washed, dried out, packed in newspaper, boxed and put away in
the warm environment of a house during the cold winter days.
the only stuff that I leave in the cold of winter are plastic bottles of Hydrochloric Acid, anything else, especially glass bottles and anything that
could be affected by corrosion, is strictly kept indoors. If you don't have room for it, the only thing I can say is make room for it somehow!
I feel your pain though, that looked like a massive condenser!
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its the dual-coil condenser on a buchi. I left it hooked up to the water recirculator (inside an unheated outside building), with the water turned
off, but it didnt drain and I didnt think about it at the time. I only got back home today and found it "not in working order".
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Haha, what a shame! How much will a replacement cost for your model . . .can a very good glassblower somehow mend this one somehow?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Mending it will be out of the question, it'd be far too tricky. The best that could be made of it is to salvage the taps and joints. Not very nice to
laugh at anothers misfortune really. I have a friend who is helping me procure a replacement. Failing that I'll save for a new rotavap.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I beg your pardon?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If you insist I explain...
http://www.thefreedictionary.com/ha-ha
Check it out.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I only got back home today and found it "not in working order".
This is what I meant as funny.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
INORGANICUM
Harmless

Posts: 11
Registered: 12-3-2012
Location: United Kingdom
Member Is Offline
Mood: GOOD
|
|
£595.00 hotplate/stirrer, after 4 can's of special brew I decided to test out my new purchace and boil a flask of H2O, I plugged it in and turned it
on, At the same time I remembered it was from the US.
Just as I flicked the switch with no voltage reduction in place. POP with a puff of smoke it went. I still can't believe my stupidity to this
day.
I couldn't possibly remember the amount of glassware ive broken, but reflux condencers is my forte for breaking glass.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Nasty, Inorganicum! Was there no protection on the circuitry in your hotplate/stirrer . . .can it be replaced, or is it under some kind of warranty?
That cost seems to imply that you got it new. Personally, I once bought a US-plugged 20V adjustable bench power supply for electrolysis etc. . . I was
only informed of its origin when it arrived and I had to order an adapter from eBay to suit. It does however mean that I can usually buy any bit of
electrical equipment from the US now if needed, which is a nice feature for the lab . . .diverse electrical sockets, I call it
Personally, I can also report the resignation of a 1L 19/26 RBF from service today. . . .my fault I guess for dropping it when I fell over the step
down into my lab
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
A $600+ stirplate/hotplate and it was not fuse protected? I don't own a hotplate myself (I'm in the process of making one), but most things are fuse
protected these days, you know, in case someone had too many beers and decided
to plug it into an outlet and let twice the current run through that unfortunate piece of macinery and decided
to plug it into an outlet and let twice the current run through that unfortunate piece of macinery
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
i used to have alot more accidents than now.
when i was 15-16 i worked in the living room quite a bit, and i spilled quite a few chemicals on the VERY expensive carpet. the worst ever was when i
dropped a 250 ml erlenmeyer (luckily only 50 ml full!) of bleach and something else on it! luckily the glass didnt break, but i threw 4 Gal. of water
on it and then dried it out, i got away with a mild discouloration. IMPORTANT LESSON: don't work on an expensive carpet!
and the next bug incident was when the cleaning lady spilt 2 Gal. of high grade navy paint on the ash floor, it took hours to remove.
all above information is intellectual property of Pyro.  |
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Nasty, Pyro!
Yesterday I was removing a stir bar from an Erlenmeyer . . .the magnetic field took the neodymium magnet out of my hand towards the stir bar inside
the flask, shattering the outside of the glass. Nasty, really, as it was holding some aqueous sodium hydroxide!
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
OctanitroC
Harmless

Posts: 21
Registered: 20-4-2012
Location: Near the nadir of a triangular state
Member Is Offline
Mood: Reduced
|
|
My hand a few days after learning the hard way that NaOH+NaCl has a nasty combination of low surface tension and good solvating properties. No sir,
not doing that flame test again. And getting a better pair of gloves.
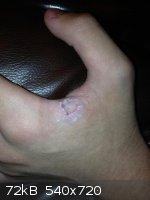
And then I discovered this charming young man had stolen my kidney!
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
thats even worse, were your hands soapy?
all above information is intellectual property of Pyro.  |
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
For a while, yes . . .I was luckily wearing gloves, but I still ran to the sink and flushed my skin thoroughly for about 15 minutes.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Teen Chemist
Harmless

Posts: 42
Registered: 27-4-2012
Member Is Offline
Mood: Effervescent
|
|
Hexavalent that would really have ruined your day if you werent wearing gloves.
I have in the last 3 monthes broken about 6 test tubes a 100 ml beaker and an Erlenmeyer flask.
Luckly the test tubes were cheap probably why they broke so easily. I droped the beaker from about 3in and it broke. The flask wasnt my fault a
spectator bumped it.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Hexavalent  | | For a while, yes . . .I was luckily wearing gloves, but I still ran to the sink and flushed my skin thoroughly for about 15 minutes.
|
What was the concentration of your NaOH solution?
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I think it was about 20ml of 0.1M standard sodium hydroxide . . .not too nasty, but still something I would prefer not to have all over my hands.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
flip flops are bad. once upon a time as a yute i thought i would try electrolysis on a molten bath of NaOH. i went to adjust the electrodes and as i
pulled one from the bath, a blob of molten NaOH dripped on the top of my left foot. no solution here it was pure NaOH. i still have a very deep and
rough scar about the size of a nickel. live and learn eh?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I just had one of those days: watch glasses and 5 ml beakers all over my benches and tables trying to find a solvent or solvent pair to recryx my
intermediate product. It put me in a frustrated mood. Trying another solvent paair and watching polymerization begin along with the unexpected
develpment of two phases didn't prepare me for having one of my two female 24/40 stoppers jump off the evaporator smashing the hard-to-find stopper
along with a beaker on its way down. This was so inspiring i decided to catch up on washing my glass and managed to break a couple more beakers. A
seried of little things like setting up a filtration and finding no paper to fit the chosen Buchner. Giving myself a lecture/pep-talk on the value of
optimizing work-ups... lets see the reaction took three days of which 2.8 days were refluxing the first solution with a Dean-Stark. The next three
steps went as planned; evaporate, wash, extract, dry, evaporate, check with TLC oh boy now comes the fun! Maybe I'll try DMF next. Acetic acid
dissolves it in any concentration.. maybe something simple like ether/acetone. I hadn't tried that pair yet (had to crack open a new can).
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Eliteforum
National Hazard
   
Posts: 571
Registered: 18-11-2002
Location: United Kingdom
Member Is Offline
Mood: Enjoying the journey
|
|
^ PPPPPP!
Proper Planning Prevents Piss Poor Performance!
All that glitters isn't gold.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Heating a non-Pyrex test tube, and cooling it down in cold water. The test tube cracked open...
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
had a one liter bit of pyrex glassware shatter on me boiling down a bleach solution to make chlorate. fortunately no glass on or in me but some burns
from the boiling fluid and steam. face and left arm. good thing for goggles. eyes are safe. no idea why it broke. i didn't move it or slosh the
liquid. didn't bump it with anything. just time for something to happen i guess.
[Edited on 17-7-2012 by Rogeryermaw]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Oh no im always worried when this thread gets bumped up again
Is it a real pyrex beaker? Is it a real hotplate?
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
| Pages:
1
..
12
13
14
15
16
..
38 |