| Pages:
1
..
12
13
14
15
16
..
19 |
1281371269
Hazard to Others
  
Posts: 312
Registered: 15-5-2009
Member Is Offline
|
|
We did pretty dull stuff in GCSE chemistry, I think the most exciting was heating Mg in a crucible until it popped, that was back when we were about
13. We did H2SO4 / Sucrose in triple i.e. for a class of about three people. But supposedly A level stuff is a lot more exciting (I've had three
classes so far).
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
UGHH!! I was considering going back to college and just getting a Chemical Engineering degree. Man, I do not know if I can survive nothing but
bookwork!! But my ebay is doing well and I should have much better home chemical setup in the not so distant future!
Fellow molecular manipulator
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
Chemistry in 1990 is a while back; things change in 10 years let alone 20.
The glass aspirator that was linked is of a different design( I linked it in my earlier post lol) and I asked whether the different aspirator designs
make a difference in vacuum pulled.
Does anyone know?
|
|
|
1281371269
Hazard to Others
  
Posts: 312
Registered: 15-5-2009
Member Is Offline
|
|
I wonder if someone could sort something out for me about this aspirator stuff:
I've heard two descriptions of the way it works. The one that seems to make sense to me is that the tube is connected with an airtight seal to the top
of a full pot of water. The water is run out. The air has to support the water and its pressure is greatly reduced, creating a soft vacuum.
The other one is about water running out at a certain speed somehow creating a vacuum...
I've done some searching on google etc, and it hasn't cleared up the issue.
|
|
|
ammonium isocyanate
Hazard to Others
  
Posts: 124
Registered: 13-7-2009
Location: USA - Midwest
Member Is Offline
Mood: sick 
|
|
It's called the Venturi effect.
Basically, a fluid moving at high speed pulls fluid around it into it's stream, propelling it in the same direction (this isn't really a very good
description, I can post a sketch if necessary that explains it better). So in an aspirator, the moving water pulls air along with it, out of the
chamber and into the atmosphere. Thus a vacuum is produced.
The same effect is observed in combo-type snowguns. Nucleation streams consisting of tiny ice particles are sucked up by water shot out of nozzles at
high pressure, causing the water dropletsto freeze around the ice particles (if it's cold enough).
On the subject of aspirators, does anybody know how good the aspirators from pelletlab are? The're pretty cheap, so the're are great deal if they
work well.
|
|
|
bilcksneatff
Hazard to Self
 
Posts: 54
Registered: 11-11-2007
Location: Maryland, USA
Member Is Offline
Mood: Sulfuric
|
|
Quote: Originally posted by chloric1  | UGHH!! I was considering going back to college and just getting a Chemical Engineering degree. Man, I do not know if I can survive nothing but
bookwork!! But my ebay is doing well and I should have much better home chemical setup in the not so distant future! |
That was high school chem (In fact I'm still in high school, this is my last year). College chem should be much better than that 
[Edited on 13-9-2009 by bilcksneatff]
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
Guys, so what about this aspirator design www.pelletlab.com/filtering_kit
As compared to a standard design
www.carolina.com/product/filter+pump+or+aspirator.do?keyword...
Any difference?
Cheers
[Edited on 04-07-09 by uchiacon]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Yes... they aren't even the same items. lol
One is a hand pump, the other is an aspirator...
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
Scroll down the page and click on the weird looking glass thing.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
I've never used a glass aspirator of that design, but for $6.95 I doubt you can go wrong. The metal aspirator from Carolina seems quite reasonably
priced and they claim it's very efficient. If I were in the market for an aspirator I would probably go for either one of those. The glass one may
be easier to connect to your water supply unless you happen to have a faucet threaded to mate with the metal aspirator, but connections can usually be
improvised with parts from your local hardware store.
|
|
|
uchiacon
Hazard to Self
 
Posts: 87
Registered: 3-7-2009
Location: The Jungle(neezeelund)
Member Is Offline
Mood: Financial
|
|
heres a really cheap one from international peeps. $20US inc shipping. I think I might go for the metal one though..
www.onlinesciencemall.com/Shop/Control/fp/sret/1813212221162...
And for NZers, heres a place in Auckland that sells metal aspirators for $54 and $78.
www.deltaed.co.nz/
[Edited on 04-07-09 by uchiacon]
|
|
|
3287
Harmless

Posts: 5
Registered: 24-12-2008
Member Is Offline
Mood: No Mood
|
|
Was any progress made with producing N2O5 from NO2 + O3? It sounds like a remarkable way to produce strong nitric acid for those of us without access
to nitrates or sulfuric acid.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
2c worth on stuff that will/won't corrode in the presence of H2SO4/HNO3 (H2SO4/Nitrates too??) for making your own retort, or equivalent.
From this link:
http://www.corrosion-doctors.org/Why-Study/Right-material.ht...
Not too sure how to interpret the diagram. They say 'the shaded zones'. They should simply say, the zones? (five of them).
Zone 2 is what would interest the Nitric acid maker (via Sulphuric + Nitrate).
Cast iron pot/cauldron welded (using cast Iron rods) to an old cast Iron down pipe from spouting (that' guttering for the US audience  ) )
Just realized, it says nothing about temperture.
Dann2
CALLING ALL MALE BEE'S, GET STIRRING THOSE CAULDRONS........
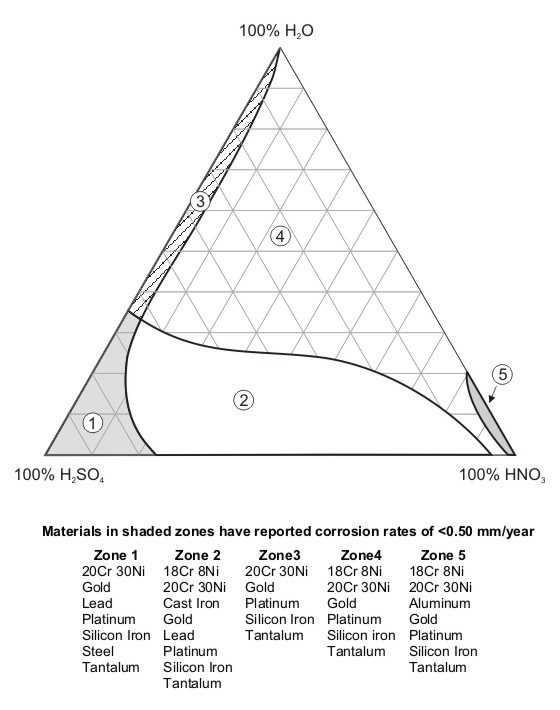
[Edited on 21-12-2009 by dann2]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Silicon steel pots are used to recycle sulphuric acid from spent nitration acid---unfortunately they occasionally fail, and catastrophically, with the
result that boiling H2SO4 enters the furnace. . .
I think I'll stick with glass; it's worked perfectly well, so far!
[Edited on 21-12-2009 by hissingnoise]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by 3287  | | Was any progress made with producing N2O5 from NO2 + O3? It sounds like a remarkable way to produce strong nitric acid for those of us without access
to nitrates or sulfuric acid. |
N2O5 is produced electrolytically too; it's fairly complex but is cheaper than N2O4 oxidation by O3!
Producing dry O3 in sufficient quantity is problematic and collecting enough N2O4 isn't easy either.
If one had big bucks it could be done but there might be other, er, distractions if you had money to burn.
Buying out LLNL fr'instance---or making porn flicks!
But it looks like us mere mortals are stuck with KNO3/H2SO4 for the foreseeable future.
If you can't get those, you've got a real problem. . .
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
You can buy 38% nitric acid by the litre as pH Down in the UK.
It is used in hydroponics and is good to use as dilute nitric acid.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by 3287  | | Was any progress made with producing N2O5 from NO2 + O3? It sounds like a remarkable way to produce strong nitric acid for those of us without access
to nitrates or sulfuric acid. |
You would have to be a bit mad to go down this route. Both ozone and nitrogen dioxide are toxic and corrosive.
N2O5 is almost unbelievably nasty. It will explode on its own, it is corrosive and it is a very powerful oxidiser that will explode on contact with
some materials.
At one time it was used as a nitrating agent but it has now been replaced by the far safer and more stable NO2 BF4.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I know N2O5 will decompose quickly at room temp., I could be wrong, but I don't think the decomp. is explosive.
It's fairly stable in DCM and HNO3 and on its own at and below -60*C.
38% HNO3 needs a lot of H2SO4 if you want strong acid, but it's a lot better than nothing.
http://www.jstor.org/pss/53948
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
N2O5 is pretty safe in solution. The pure material is nasty.
It is just like nitrogen trichloride, pretty safe in solution in carbon tetrachloride, but a nice little yellow pool of the neat liquid is a definite
no no....
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
N2O5---> 2NO2 + O ---not a detonation; NCl3, though *does* detonate, sometimes apparently spontaneously.
Pure N2O5, itself, is a colourless crystalline salt.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by hissingnoise  | N2O5---> 2NO2 + O ---not a detonation; NCl3, though *does* detonate, sometimes apparently spontaneously.
Pure N2O5, itself, is a colourless crystalline salt.
|
You mean;
2N205 -> 4NO2 + O2
Two moles of solid to five moles of hot gas, that is a detonation in anyone's book.
N2O5 runs around as NO2 NO3, the salt that you describe, but it also enjoys life as NO2ONO2.
Give the latter form a rubber crumb or two to bite on and it will take your hand off!
[Edited on 22-12-2009 by ScienceSquirrel]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
ScienceSquirrel, N2O5 is an oxidiser like N2O4 and is no more explosive than that substance.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
A quick web search will reveal that N2O5 is a lot less stable than N2O4.
N2O4 is hypergolic with some hydrazines and will form explosive mixtures with some things.
N2O5 is in a different league, it explodes on contact with quite a few organic materials and some inorganic ones.
I cannot find a primary reference to it exploding on its own but there are plenty of secondary ones eg http://en.allexperts.com/e/n/ni/nitrogen.htm.
I would also point out that the structures are totally different, N2O4 is a dimer while N2O5 is not.
There are plenty of gotchas in chemistry where extrapolating the properties of one compound to another lands you in the doo doo.
Oxalyl chloride is often used as a model compound for phosgene and some of the chemistry is genuinely homogous but if you knew about oxalyl chlorides
toxicity you just would not predict how toxic phosgene was.
Oxygen and ozone are both oxidisers but the difference of degree is huge. Condensed oxygen will just ignore a bit of silicon grease, condensed ozone
will explode!
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I still contend that, by itself, N2O5 is not an explosive. . .
So, find a primary reference, and I'll concede the point!
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I think we will have to agree to disagree, I have searched for a primary reference and failed to find one.
If I was planning on making it I would have a very careful literature search though.
One eye good, two eyes better 
|
|
|
| Pages:
1
..
12
13
14
15
16
..
19 |