| Pages:
1
..
10
11
12
13 |
WGTR
National Hazard
   
Posts: 972
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Thanks for the pointers. I'm trying water-cooled copper capillary tubing right now, to see how that holds up. After all, that's how it worked in
industry. The electrodes are holding up somewhat, but there are a few things that I need to adjust in the arc. I'm not spreading the arc far enough
yet, and the current is being concentrated too much in certain areas of the electrodes. Aside from that, the water is keeping them cool. I'm using a
DI water supply right now, with no recirculation (just runs down the sink). Water runs through the positive electrode, and exits through rubber
tubing to the negative electrode. The water is not even warm as it exits.
As I ponder my previous post, I realized that I completely missed something obvious. In the original Birkeland-Eyde reactor design, the supply
voltage was 5000VAC, at something like 40A. This is, of course, for a full sized reactor. The current limiting was provided by inductive ballast.
This inductive ballast causes the arc voltage to swing not only below, but above the supply voltage. Think of the arc as if it were a MOSFET in a
flyback converter. When the FET is on (low arc voltage), the inductor charges up. When the FET turns off (arc blown out to the edge of the reactor),
the inductor dumps its energy through the load resistance. If the load resistance is very high, then the voltage across the FET (arc voltage) can
rise to very high levels, at least until the FET avalanches (arc voltage in this case may rise to 10's of thousand of volts). This is a weak analogy,
and what is happening in the arc is more complicated than this, but it's the general idea.
Up until now I have been using a 150VDC supply that is current limited to 3A, and with 30 ohms of ballast resistance. Since there is a large output
capacitor on the power supply, the voltage does not rise fast enough when the supply goes into current limit. Using the ballast resistor gives a much
more stable arc for this reason. Arc spreading is accomplished with an electromagnet that is driven from 10-60Hz AC. The electromagnet core is
ground to a point, like in the original reactors. Since I am using a ballast resistor, not an inductor, the arc voltage can never rise above 150V.
Resistors don't store energy like inductors do.
The next order of business is to calculate what kind of ballast inductor that I need. It will be determined by load current, electromagnet frequency,
and efficiency, among other things. Hopefully my 1cm diameter arc will spread to a 10cm arc with this modification.
Once I get certain issues ironed out, I'll probably start posting pictures.
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
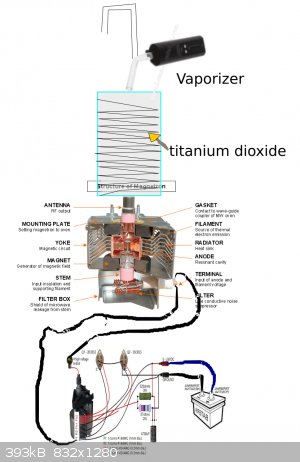
Will it work?
the titanium dioxide on carbon will take the hydrogen from the water ?
and the flyback can be placed in the magnetron ?
I saw that the magnetron has an input of 4000 volts, the flyback has in its output 30 000 volts, as there is no coil or electronic part within the
magnetron believe he can mutiplicar this value?
please help me
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
I'm going to attempt to make nitric acid in a few months with a zvs driven flyback transformer.
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Made it. Two minutes will let it redden litmus paper.
[Edited on 3-2-2016 by Hawkguy]

|
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Woot Hawkguy! That is some accomplishment!
Well done.
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
articles on dielectric barrier discharge
Hi I've found two neat articles that may be of interest to those designing plasma generators for both nitric acid and sulfuric acid. The articles
focus on dielectric barrier discharge plasmas as they are supposedly more efficient and less hot than open-arc typical Birkeland-Eyde reactors.
edit: and the arc being less hot allows more generation of NOx without it dissociating and being destroyed from excessive heat.
Attachment: experimental.pdf (1.1MB)
This file has been downloaded 1640 times
Attachment: SO2 removal from air with dielectric barrier discharges.pdf (1MB)
This file has been downloaded 1254 times
[Edited on 8-8-2017 by physics inclination]
|
|
|
UkAmateur
Harmless

Posts: 11
Registered: 4-8-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by axehandle  | | Quote: |
"Besides, human lungs can take a lot more than one would think"
axehandle you are without doubt a fucking idiot.
|
Can you spell "humor" and "irony"?
| Quote: |
Its very easy to get blase about NO2. Familiarity breeds complacency, particually when you havnt read the safety data.
You can breathe in NO2 and feel as if youve got away with it, normal, undamaged. Then 8 hours later, up to a day or two, you can drop dead of
pulmonary edema. At lesser doses NO2 does largely unnoticed but long term damage to the rather fragile lung tissue. This is not simply caused by the
acid NO2 forms as it dissolves with water, its a very reactive free radical - also why its paramagnetic and dark brown.
|
Well, I've read up on it now, after a very nice person warned me without calling me "a fucking idiot". Apparently it is much more dangerous than I
previously thought. I know that now. I'm not senile yet.
You might want to save on you "fucking idiot"s's. People tend to stop reading what you write after you start the childish name-calling.
[...snip...]
| Quote: |
The thing about people that taste chemicals or test for high voltages with their fingers or hang around neer lit fuses, when everything goes fine
people say 'hes, crazy' 'he must be insane' and then grin, and when things go wrong they say 'Oh shit, that was unlucky' or 'he shouldnt have done
that' when what they really mean in both cases is 'what a fucking idiot'.
|
I don't test for high voltages with my fingers, nor do I use fuses at all. I always (fior my rocket engine tests) use electrical ignition with a very
long cable hooked up to an apparatus with TWO safety switches.
Nor will I ever "taste test" nitric acid again. Have you heard about learning from one's mistakes?
| Quote: |
While in the long run it makes no difference if you kill yourself or when, there will be a lot of other people reading this thread that will assume
you know what you are talking about. If you adopt a lax attitude to NO2, they will assume this is acceptable. It isnt.
|
It's not my responsibility what other people think. And my "lax" attitude is part of my humor, which you obviously don't get. You must be a very
boring person, OR there's something wrong with my humor.
[...snip...]
| Quote: |
Aparently high school physics is letting you down. An arc is a low conductivity path through the air, its low conductivity becuase its hot and
(partially) ionised which the current maintains. Hot air rises becuase its less dense than the surrounding air and when it rises it pulls the arc with
it since this is the path of least resistance. The arc is rising with the air, not through it. Trimming the ladder until the arc does not break solves
a lot of problems, and combined with a static vertical magnetic field should increase yeild substantially (particually concentration in air making it
easier to produce better nitric).
|
That's why the air is pumped in TANGENTIALLY, so that the arc will contact more (turbulent) air than if it where intruduced AXIALLY (w.r.t. the
ladder).
I have already discarded, dumped, ditched a static arc, since it would require electrode cooling.
| Quote: |
"I AM going to "recycle" some of the NO2 by feedback. "
No, dont do this, it would be bad.
"Why?"
Air passing into the arc comes out with a concentration of nitrogen oxides that does not depend on the amount going in. Its wasted NO2. Aditionally
people seem to get better results if the air going into the arc chamber is dry.
|
Fine. Won't do it then. No problem. Actually, one less problem.
| Quote: |
You might want to take heed from the death of a NST in jacobs ladder config particually.
"The company manufacturing this particular NST gave me their word that it would work in a jocob's ladder config."
Read the thread on roguesci. While it will certainly work, how long it will live is more the question. I came to the conclusion that the constant
sparking might well be generating fast voltage spikes (eg from inductive kick) that degraded the insulation over time. Its also possible it simply
overheated from the neer short current.
|
Time will tell.
| Quote: |
"I'm not intending to make explosives. I'm very afraid of explosives. I AM, however, very interested in rocket fuels."
There is not so much of a difference. What fuel/oxidiser are you planning to make with the nitric acid?
|
There is a very large difference. Rocket fuels deflagrate, explosives detonate. I was planning on trying out NC to start with.
| Quote: |
For the record, I do not have a 'problem' with your project. I hope it succeeds. You are quite entitled to tread your own path, make your own mistakes
and we will learn from it either way. I will try to help, you can listen to my suggestions or not, if something is covered well elsewhere I do reserve
the right to simply point you to it rather than type it all in myself. If you state as facts things I know to be wrong I do reserve the right to
correct them and last but by no means least, if you tell people things are safe when they arnt, if you tell people something is doing less damage to
them than they think - when you havnt read the information and very particually when it relates to NO2 - then I do reserve the right to call you a
fucking idiot.
|
Everyone states as fact things that are wrong. It's the listeners responsibility to determine the truth of the statements. I could state that it's
safe to jump out a window --- that does not make my fault if someone tries it.
And if you care to point out exactly <i>where</i> I've stated that NO2 is absolutely harmless, I'll accept the ad hominem title "fucking
idiot". Mostly because sometimes I'm an idiot, everyone are, but also because I have a girlfriend, which means I'm occasionally fucking.
Does you hardware not support the concept of irony? And why are you so fond of the expression "fucking idiot"? Is there something Mr. Freud would find
interesting here?
[Edited on 2004-2-24 by axehandle]
[Edited on 2004-2-24 by axehandle]
[Edited on 2004-2-24 by axehandle] |
It's posts like this ^^^^ that really make a 'like' or 'rep' button a necessary addition to the forum soft ware in my most humble of opinion..
I don't want to waste bandwidth (and thus the site owners money)
So rather than quote a whole post.. Just to say I strongly agree with the posters opinion/sentiments..
A simple thumps up could suffice...
A constructive critism is all. Hope it doesn't over step the mark with me being new n all..
Regards
E2a I'm sorry but the quote in my box and the one appearing here are different?
If a mod can help? Or just delete? Many thanx.
[Edited on 8-8-2017 by UkAmateur]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2834
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
NO2 is a toxic gas. However, it is less toxic than chlorine or ozone, which are frequently recommended on this forum. According to this paper:
http://www.env.go.jp/en/air/odor/measure/02_3_2.pdf
The odor threshold for NO2 is 0.12 ppm, and the PEL is 5 ppm with an IDLH of 20 ppm. Obviously you don't want to be pushing up against the IDLH and
you need to use a respirator but it's not exactly nickel carbonyl, and the smell is an available warning that something is wrong. NO2 is infamous
because it's easy to produce it inadvertently from common materials or when trying to do anything with nitric acid (particularly dissolving precious
metals).
|
|
|
j_sum1
|
Thread Split
25-7-2018 at 02:58 |
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
peculiar salt in residue
Has anyone noticed a white salt left behind after evaporating all the nitric acid produced by the Birkeland-Eyde method?
This salt is white but it turns ammonium thiocyanate solution red! Seems like iron impurities but also other properties show it is not. If anyone is
interested in further investigation what it could be, tell me to share more info.
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Dear Colleges, this is my first post and so pls. forgive me to "bore" you with a short introduction of myself. Come from central Europe and work in
technical area in automotiv and chem is my hobby. I was behind this Birkland process for several years an so found your board with very interesting
designs and approaches to that topic. So I decided to register and learn more from you.
With the high voltage jacobs ladder designs I ended up with too little success (too less hno3 production) per time so I stopped investigating. But
recently I found some article about nox production in an cold plasma (dbd) discharge with some kind of dielectric that is also catalytic (tungsten
oxides mixed with aluminium oxides)
Has anybody of you heared about that? This kind of design does not have electrode degradation and for the hv supply a flyback from an old b&w
television ore something like this will do and the high frequency of some 10khz will also be helpful to increase the yield.
Loking forward to your comments
(and sorry for my English  ) )
Attachment: TUe-Plasma.pdf (1.9MB)
This file has been downloaded 771 times
[Edited on 23-4-2020 by FranzAnton]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
Theoretical with under pressur sparkling you would have the gratest yield. Haber wrote abaut it around 1907. I ve read they had the same idea with the
electicity from the wind mills in North Germany. Lower pressur sparkling should double the yield.
[Edited on 23-4-2020 by Alkoholvergiftung]
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
I also read that, but at that time they had no idea about cold plasma physics.
Anyway I woud give the "old style" design another (last) chance, but with a different design regarding the arc unit.
High volatage unit DC which is charging a powerful capacitor which can reach 15kv. Replace Jcobs ladder to a normal 2 electrodes with fixed length
spark gap which fires at 15kv. After each arc the cap can recharge until the next discharge. Powersupply an capacity I would design in that way that
10 arcs a second will be possible.
The electrodes placed in a narrow quarz tube (approx. 5--8mm inner diameter)
tig-Tungsten electrodes. The air will stream coaxial to the electrodes an will cool them and transport the reaction gases fast into cooler areas. Also
the quarz tube has to be cooled outside with water (isolated double mantle)
The power supply is in that case more expensive (door nob capacitor) which can stand the high discharge currents.
I think this desing helps in faster cooling of the reaction gases which directly corresponds to higher NO yields.
With a given capacita, discharge voltage an frequency you can calculate the average power the high voltage system das to deliver, but a system below
500 watts limits the fun a lot 
[Edited on 24-4-2020 by FranzAnton]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
It s only good when you have cheap electricity. I think an Transformer with 6kv max would be better. There is no Need for so much voltage. For the
stove i think an mix of sand and gypsum would be an good Isolator and heat recistent. Cooling the gases obove the stove should Highend the yield too.
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
lets forget the costs for electrizity for private project for a while. I need to have a better production rate and the NO production correlates with
the volume of the discharge and this becomes grater with 15KV. But you are right several 6kV arks will do the same.
I will not stick to the 15kV. Depends on the material (transformer, caps. e.t.c) what I can utilize. But I consider the 15kV as a kind of max. voltage
which I can handle without too much restrictions. Waht I also had in mind is, that the stored energy in a cap. rises with the square of the voltage.
Further it's important to have a very short time of the arc (and that's depends of the capacity) so if you need extrme hot and short and energetic
arcs you have to optimize that 3 parameter. Do you agree?
I forgot to mention an interesting detail that an extreme ultraviolett radiation generated by very hot arcs contributes also to the NO production.
So my opinion is that the jacobs ladder has a lot of limitations for higher production rates, but the advantage of it is the simple design. The
original industrial scale Birkland-Eyde ofen generates 1% NO in the exhaust gas. The question is, if it is possible to reace 1-2% also in an
donwscaled experiment like it can be done at home? I think for a much smaller apparatus a completely different arc design is necessary.
Do you speak german too (cause of your nickname) 
[Edited on 24-4-2020 by FranzAnton]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
Yes i speak german too. Is there not an Energy loss with 15kv because of radioaktivity? I ve read high voltages like in Thunder storms produce
radioaktive reaktions.
They wrote that the Schönherr Stove had an eveciency of 5,5%.
They blew air from the Bottom of an pipe like stove i think one Elektrode was on the Bottom the other on the top with an arch lenght of over 1m. But
you Need an lot of Compressed air.
You want to use Capicators like the Marx circuit?
[Edited on 24-4-2020 by Alkoholvergiftung]
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Radioactivity in that case is new for me. For 15kv I would not use a cascade. I would use a kind of flyback transformer loading that cap. But I am not
clear now what exactly my next attempt will be. I found an interesting work about a GlidingArc reactor. Which basically is a kind of jacobs ladder in
a flat surface design which looks very promising to me and which is driven by a normal 60Hz transformer with 5kV. The flat design helps a lot with
cooling fast. I think the arc reactor itself is the cheap part, but the following oxidation part is the tricky thing where NO --> will becom NO2 if
money is not so relevant (hehe) my idea would be to buils a "reactor not as a vessel as usual. I would use (pls. don't laugh) 200m PTFE tubing as a
coil with 12 inc diameter where the exhaust NO gas and extra oxgen (or clean air) have time to react while constantly flowing in that 10mm inner
diameter ptfe hose. 200m of that kind of tubing costs here 600$ ..thats a lot and have to be considered very carefully. The reactor design I have
"stolen" in the web 
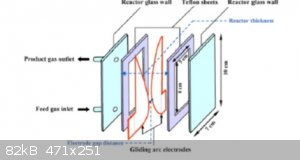
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
The Ozone Generators from Amazon are an good source 6kv 100W.
If you work on small skale.
I would fill the bottle with claypott or brik pieces and moisten them.Pumic Stone should work too.You have an much higher survace erea.
http://dingler.culture.hu-berlin.de/article/pj117/mi117mi06_...
For the Radioaktivity part.
https://www.weltderphysik.de/gebiet/erde/news/2017/gewitter-...
15kv i think is the starting range for that Reaktion.
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Thank you for those interesting links. I did not know that and so learned something new. The 100W HV supply is too small for my needs. I am looking
for a transformer in the range of 500 to 1000W. What do you think of the idea of the PTFE hose as a gas reactor?
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
You have an lots of heat. I think it will melt. I would use the gyps sand mass in an Copper pipe as an chamber.Or Schamott.
|
|
|
MarkRob
Harmless

Posts: 6
Registered: 20-4-2020
Location: UK
Member Is Offline
|
|
Has anyone tried microwave plasma? It looks a bit tricky to do well - needs low pressure around 50millibar, which would make NO oxidation take many
hours, so the NO product would need to be passed through the vacuum pump to an oxidation/absorption chamber at 1bar.
Also it looks like efficiency is quite a lot higher if the O2 content is around 35% or higher, but this could be achieved using an oxygen concentrator
(but they have all be bought up due to coronavirus...)
One of the advantages of Birkeland-Eyde or microwave equivalent is lack of H2O contamination of the product, so it would be possible to freeze out
pure N2O4 without contamination.
Interesting paper attached, they get 2.5 times the energy efficiency of Birkeland-Eyde without using any catalyst.
Of course, magnetron efficiencies are down around 60% or lower, so the actual efficiency gain is small, but the yield is quite high and there are no
electrodes to wear out or contaminate the product.
Attachment: ajp-rphysap_1984_19_6_461_0-microwave-plasma.pdf (682kB)
This file has been downloaded 695 times
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
hm that’s right, after the arc I have to put a glass tube with extra cooling to come down to 200 C which is good for PTFE.
For the concentrating the HNO3 I will test the following way:
HNO3 of round 50 to 60% is needed first this can solve a lot of NO2 gas. While solving it became deep dark green. If it’s saturated, I put the glass
bottle in an pressure vessel and press pure oxigen 20 bar on that solution. So long until no more Oxigen is absorbed. It is possible to convert a
diluted HNO3 saturated with NO2 in that way into a concentrated one.
A bit an effort it is to make that pressure vessel from stainless steel which can stand 30 to 40 bar (some extra for savety)
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
to deal with magnetron for continuous max power is much more sophisticated and the yield can also be high with a normal arc design if you can manage
to cool down the gases fast enough.
With the GlideArc design I will manage a fast cool down by a turbulent flow design. On the smallest point of the arc gap I will feed in the air with
high pressure 18 bar from a fridge pump. To limit the air flow I use a glass capillary. So I get a very fast Air stream in the sparc gap. Because the
arc gap is flat, I also can cool the quarz glass sides, so the faster the gases can be cooled down, the higher is the NO content.
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 198
Registered: 12-7-2018
Member Is Offline
|
|
30 to 40 bar is an very high pressure. Somewhere here wrote someone Silicagel can absorb 70% NO gas. May be it s usefull you can heat it later and the
Temperature is lower so ist more stabel.
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
silicagel will only help in concentrating NO2. I described to solve. NO2 in HNO3 then with the oxygen pressure you can convert the NO2 with the rest
of water in the HNO3 into HNO3 so it will be concentrated by this way.
|
|
|
Belowzero
Hazard to Others
  
Posts: 175
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
I made a video about my birkeland eyde reactor, or Pauling reactor.
It's a work in progress, intended as more than just a POC.
The primary part of it seems to work well at this point, I am still playing with some ideas inspired by long forgotten patents but for now this is not
where the focus lies.
The next challenge is to build an efficient scrubber, perhaps using a spraying device of sorts as is used by several industrial processes.
If I can get something that is capable of running for days on end I might buy a few second hand solar panels to see if I can run it on those, nitric
acid from air,water and sunlight is the final goal here.
Any feedback is appreciated 
https://www.youtube.com/watch?v=nwmXDqePNrs
|
|
|
| Pages:
1
..
10
11
12
13 |