| Pages:
1
..
9
10
11
12
13
..
28 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My aluminum fume hood has been in home chemistry service for 5 years now. I decided that it's time to take the two baffles out and inspect behind
them and the mouth of the 8" diameter PVC outlet.
Everything was clean on the back of the hood (epoxy painted), and the PVC outlet was clear with no corrosion, particulates, or other debris. The only
thing there was some soot from my attempts to make coke from coal. I had purposely left the two baffles as bare aluminum as an experiment. They did
suffer some superficial damage from NaOH solutions and fumes. The baffles are now being painted with epoxy prior to reinstallation.
Below are pictures of the hood without the baffles and the baffles being epoxy coated.
 
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
Here is glasware i had in my room. Soon i will make real lab with fume hood and more glasware  
[Edited on 16-8-2010 by Mildronate]
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
new lab
september is coming up and i have a few dollars to spend. i will be constructing my lab soon and i am curious as to advice or help that any of you may
be able to offer. My list so far includes roughly $1500 to use for this purpose. i found a 8'x10' storage building for $250 and a glassware set for
$300 that includes 2n3 neck boiling flasks, a vigreaux condenser, a liebig condenser, still head and several adaptors and keck clips, and a heating
stirrer for $130 that gets to 320 Celsius. a will build my fume hood and have the price of it down to about $150. of course i will run power to the
building but i already have the materials for that and more lighting than i know what to do with. running water is on the list but due to construction
difficulties it will have to come later. any suggestions at this point will be greatly appreciated and well received. thank you all.
|
|
|
Gruson
Harmless

Posts: 30
Registered: 22-10-2005
Location: Southern Netherlands
Member Is Offline
Mood: Creating!
|
|
I just bought two vacuum gas manifolds (schlenk lines) with intention of keeping one of them and selling the other, which would result in a free
schlenk line.
But i am not setteled on which one I will keep. One is with all Normag teflon valves, and one is 50% normag valves and 50% glass valves. I like the
glass valves for that you can see in which position the valve is, but I have heard that teflon valves seal off better. A person on a Dutch forum
mentioned that you could open the teflon ones more graduately. What do you think?

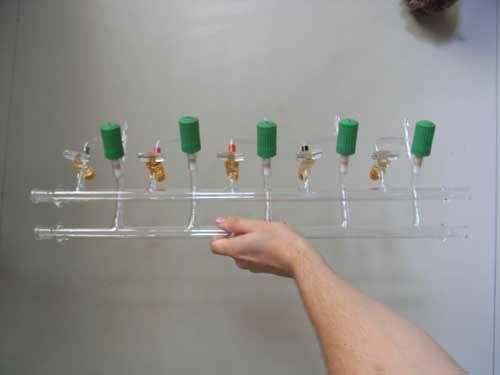
[Edited on 8/20/2010 by Gruson]
[Edited on 8/20/2010 by Gruson]
It could be that the purpose of your life is only to serve as a warning to others.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
@magpie
Great to hear an update on a 5 year old hood. Do you work with corrosive gases very often in there? The rot brass / stainless kind. If you do, the
epoxy seems to be doing a good job.
@gruson
That's a nice bit of glass, congradulations on the two!
Not got a schlenk myself, but I have read numerous posts and pages from people saying the glass stoppers used in these are usually precision ground,
better than normal stoppers. The design is also laid out to feature a better cut off from the atmosphere than a simple through hole would. Instead,
the stopper is hollow inside with one hole poking into the cavity; have a look and see if yours are like that (those green taps make me suspect it's
Chemglass, and likely rather well made if so; though I don't see their snot green logo on there).
I can only speak from experience with PTFE and glass stoppers in sep funnels (the thru hole kind). The glass is easier to adjust the flow rates of.
The PTFE has a tendency to jam because it doesn't expand and contract at the same rate as the glass around it, as others here have pointed out in
other threads.
Those rotational stopcocks don't jam as easily (the PTFE is part of a plunger on the screw thread, not a friction fit element like the thru hole
taps). If the others are the same, I expect they'll be okay. If they're the normal tap kind, I'd keep the glass.
Here's page with lots of pictures of the more specialized taps you can use as a reference on build quality (oblique stopcocks)
As he says, keep in mind that cocks like these are often ground to fit a specific female (awwww), so swapping them around won't help. I've seen people
putting little blobs of coloured dye and such on these so they know which goes where if it needs taking apart.
I have two GL45 to B24 PTFE adaptors from NDS Technologies, who distribute solely (as far as I'm aware) through Sigma; they produce all that glass
that screws together, with red screw elements (I think Klute may have a fair bit of that). The adapters cost a lot each, I seem to remember it coming
out around a hundred for the pair by the time I got them. I still had a vigreux jam so tightly into it I had to bang the PTFE cone off with the screw
cap, over a few weeks to avoid going overboard with the force.
Not only that, but the asshats didn't fit standard GL45 bottles (and they wouldn't refund them). I had to have the workshop at university spin them
down on the lathe.
[Edited on 21-8-2010 by peach]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by peach  | @magpie
Great to hear an update on a 5 year old hood. Do you work with corrosive gases very often in there? The rot brass / stainless kind. If you do, the
epoxy seems to be doing a good job.
|
Yes, I have boiled off a fair amount of NOx/HNO3. I also have made bromine numerous times, which tends to initially overwhelm my condenser and shoot
gaseous Br2 out at the vacuum adapter port. My catch pan is 304 ss and shows numerous splotches of discoloration. If they get too bad I remove them
with kitchen scouring powder, sandpaper, or a 3M abrasive pad.
I haven't inspected the blower internals (epoxy coated steel) or the 304ss outlet plenum yet. That will take more effort.
[Edited on 21-8-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Gruson
Harmless

Posts: 30
Registered: 22-10-2005
Location: Southern Netherlands
Member Is Offline
Mood: Creating!
|
|
Thanks for the information and the link. I'll keep the one with all-teflon rotational valves.
It could be that the purpose of your life is only to serve as a warning to others.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | | My catch pan is 304 ss and shows numerous splotches of discoloration. If they get too bad I remove them with kitchen scouring powder, sandpaper, or a
3M abrasive pad. |
I have a big (retired) kitchen stainless sink next to me that you may have seen in those pictures I posted in Sedit's thread. I suspect it's 304. Once
a tiny amount of the HCl(g) starts making a break for it, it rots the jesus out of the 'stainless'. I'll take a photo of it's current state soon.
I guess you're probably far better off in those terms, because the airflow means the gas doesn't have time to hang around.
Have you got a carbon scubber on that hood?
Quote: Originally posted by pHzero  | Just need some light (i was thinking of some low bay metal halides off ebay)
[Edited on 5-6-2009 by pHzero] |
Arrrr don't buy them lar... kidda, fella, lad, mate... (can you guess where I live?) 
If it already looks like a meth lab, adding halides will make it look like a stoned meth lab (the worst kind). Halides are the absolute tits for clear
vision, but they'll ruin light sensitive work as well; labs (particularly silicon and biology ones) often have red / orange fluorescents they can
switch on for that reason.
If you're a UK madhatter, I would strongly suggest you buy your lab hardware (fittings etc) from Toolstation.com. I will happily ramble on about them
and provide them with free advertising. A third of the price of places like B&Q (cheaper than the other catalogs as well), next day free delivery
on all orders over £10 before 6pm. They're open until 6. They're open on Sundays. To order in store, just go in, fill in the card (Argos style) and
they'll get the parts together for you. There is a free vending machine in my local one as well. The place will be packed with all the trade guys, who
you can get friendly with and ask for advice with weird things on. They also sell things like concentrated sulphuric, minus the detergents (which
people like B&Q have stopped selling after some dumb woman burnt her face off with it, having not read the label).
I've seen those labels on your acetic before, I have one right here actually about two foot away. They feature some remarkably shit spelling, grammar
and punctuation I think you'll agree on actually reading them. I was smiling like the Cheshire cat when I noticed it out of the corner of my eye on
mine.
I have also ordered from Mistral (sp?), but only a 5kg tub of KOH I use for the washing up. I was going to pick something up soon to have a go at
vanillin from eugenol using the #o1|) skoolZ# method, for the fun of it. As entropy says, I've had the 5kg tub for literally years, I just throw
handfuls of it in as I clean the glass, which I do on a near daily basis. It's still the same tub. And it will most certainly begin sucking up
atmosphere if I leave the lid off, I can see visible droplets forming on it within 10-30 minutes.
I'm all for a bit of mess (quite a lot of it actually), but you're going a touch overboard on it thar shipmate!
Actually;
| Quote: | | I might cycle down to homebase and get some £9.99 fluorescent tubes |
The mention of cycling to Homebase to buy delicate, long, glass items makes me think you may live quite close by. 
I'm 25, but I suspect you may be a bit younger still. Be careful with your friends at school around that kind of stuff. It'll look like a drug lab to
them already (as per your mum's opinion), having stuff lying everywhere will make it worse. You's dern wanna have the boys in blue round for no
reason, they hate having their time wasted. They've got a habit of damaging doors and harassing your furniture and fittings as well, which gets
equally expensive. Teenagers love to chinese whisper 'the kewlz about drugs n'd bombz'. 'They'll' probably be wearing black, bulletproof vests and
have guns if they visit. That's no fun at all, at 4am in the morning, whilst you're lying naked in bed. Better the nerd than the guy down £300 on a
new PVC front door and with 'terrorism' on his CV.
If anyone is bothered about that, call up the local fire brigade and police and ask them to have a look round.
If you PM me with a rough idea of where you live, we might be able to meet up and you could make use of some of the things I have. But you're not
borrowing them until you stop leaving stuff out on the grass. 
It's an open ignition source, and a very good one at that too (better than the cigarettes I keep flicking around my garage as a I work. I'm not going
to call it a lab, I've only seen one lab in this thread; I think we all know which one I'm referring to).
I had an arguement with entropy over acetone and fires. But a bunsen + solvent + heat requires a lot more care than a hotplate does.
Do a wiki / google all over the terms 'flashpoint' and 'autoignition'; they're two very different, very important things. Vapour pressure is another
one to look at. And start using the NFPA 704 fire diamond (that colourful looking 'diamond' on the wikis) instead of the dimensionless hazard
pictures.
I'm not having a go at you, they just do have issues when around solvents compared to a hotplate.
You can scavenge adjustable, fully functional, almost new surface top hotplates from the tip nowadays; everyone is throwing all their stuff away as
fast as they can swipe the credit off the cards. The widescreen TV in the bedroom is from the tip. My gamecube was given to me. As was the £600 HiFi
and the computer is the cheapest I could find.
| Quote: | Oh and my spirit burner just melted a test tube along with the Zn inside it 
|
Use glass next time. 
I'm only teasing.
I've noticed numerous things in this thread that tell me at least a couple of you are in the UK. Glad to see some Tesco's shoppers (1 in every 8
pounds in the UK is spent as Tescos)! We should have a UK madness orgy at some point.
Quote: Originally posted by The_Davster  | Nice ozonelabs, great to see there are still companies that have not given into fearmongering and will send a private citizen chlorine gas. For free
nonetheless.
This warms my heart, it really does. |
I'm not sure how much of a friendly present those are. These bottles are so badly corroded by the things in them, they're sold (even in the UK, were
we rent most cylinders) with the bottle as well, and it goes in the bin when it's empty.
The age of them also suggests they're bin material they're trying to get rid of without paying the disposal charges on.
I was asking after a 220g lecture bottle of HCl last week. £140+, and they need a £500 Monel regulator, and a university VAT code, and a chat with
the company's chemist usually (which can be very informative). So I expect those bottles are as much "deal with our hazardous commercial waste" as
good will gifts.
Very nice to have them, but the motives are unlikely so good at heart.

Pictures of my garage will be on the way, lar..... kidda......
Personally, I want to see entropy and blogfasts workspace, arrrr yea!
Careful with those roto's guys, they're what the authorities term "ultra high tech advanced synthesis equipment".
John
[Edited on 21-8-2010 by peach]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | As entropy says, I've had the 5kg tub for literally years, |
Actually my unit of time is decades, not years.
peach, it worries me that you use KOH for everyday cleaning. I use it when I need to, as a KOH/ethanol bath, but detergent works most of the time.
KOH or NaOH in the eye can cause a devastating injury, much more quickly than can acid. Be careful!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by peach  |
I have a big (retired) kitchen stainless sink next to me that you may have seen in those pictures I posted in Sedit's thread. I suspect it's 304. Once
a tiny amount of the HCl(g) starts making a break for it, it rots the jesus out of the 'stainless'. I'll take a photo of it's current state soon.
I guess you're probably far better off in those terms, because the airflow means the gas doesn't have time to hang around.
Have you got a carbon scubber on that hood?
|
I don't seem to have any trouble with con HCl. It's so obnoxious that I'm very careful with it and always have the hood fan on.
I have no scrubber. Dilution with air is my solution, ie, lots of cfm. I don't have anyone walking by my outlet as it is 15 feet in the air and on
my property. Anything you put in your vent system causes pressure drop and must be considered in the design stage.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Not conc. HCl. The gas.... 
Quote: Originally posted by entropy51  |
peach, it worries me that you use KOH for everyday cleaning. I use it when I need to, as a KOH/ethanol bath, but detergent works most of the time.
KOH or NaOH in the eye can cause a devastating injury, much more quickly than can acid. Be careful! |
Quite, I am simply lazy and go straight to the KOH when I see the road tar appear.
Most of what I do is organic, and doesn't have a lot of references. Meaning I have to routinely deal with the brown / black mess that simply won't
come off with normal cleaning. Particularly to an extent where I'd think "I'm happy to reuse that for experimental work".
Never had in my eyes, and I'm not wearing googles or taking what I would consider to be much care. The most depressing thing about KOH, in my
experience, is that my hands look 60 years older if I don't put the gloves on.
I purposefully never use household detergents due to what they have the potential to do with regards to phase boundaries; coupled
with their poor cleaning power. I'm measuring out milligrams of phase transfers to look at their properties, so having powerful surfactants and
emulsifiers around is not good at all.
But yes, if you're not used to using it, getting it in the eyes is a possibility, and a very unpleasant one (that will likely require a hospital visit
and result in permanent damage).
The worst I've had in my eyes was DCM.
To clean the glass, I start with KOH, rinse, go to conc. H2SO4, rinse, then Piranha.
[Edited on 21-8-2010 by peach]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Lazy, or just the hubris of the young. Us old farts have lost that, mostly. And entropy, being an MD, has likely seen way too much pain and
suffering already.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
Lazy, or just the hubris of the young. Us old farts have lost that, mostly. And entropy, being an MD, has likely seen way too much pain and
suffering already. |
I've been doing it for years on a daily basis and never had a single problem, but for the ancient hands.
Not a single time have I had it in my eyes. I have more problems cutting up Chilli's for dinner and then wiping my eyes or touching my penis.
It's not the chemicals causing the problem, it's who's mixing them together. Now that I've been doing it for years, I handle it like it's washing up
liquid. Piranha and HF is were I start thinking.... "uh oh....."
As I edited above, the HCl I'm working with is the gas, not hydrochloric. The gas is far worse in terms of corrosion; normal keck clips fall to bits
in a minute or two (that's why Klute is using PTFE clips).
If you're not used to cleaning with at least weak KOH, there is a good chance (I'll admit) that you'll spray it in your eyes or otherwise burn
yourself. What worries me are the videos on youtube suggesting Piranha and HF are in the same domain.
I've purposefully burnt myself with 35% H2O2 Piranha and inhaled HCl(g). I still hide when it comes to using it on a flask I've already KOH'd, rinsed,
H2SO4, rinse'd etc, and HF is worse again. Those videos, trying to equate the two, are a joke. Going from a rinse with water or some dish soap to one
of those two. Water is essential for life, a lot of soaps are safe for being in my eyes, dilute KOH stings a bit on open cuts, the others unexpectedly
explode or kill you.
[Edited on 21-8-2010 by peach]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by peach  | | I have more problems cutting up Chilli's for dinner and then wiping my eyes or touching my penis. |
You
might be a chemist if ... you wash your hands before using the bathroom.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Piranha'ing my hands is about the only time they get a clean.
You've all been warned if you come round for dinner at any point... 
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Thanks, peach. I really needed to think about
A: KOH + Penis
B: YOUR Penis.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I'll send you a picture of both for £5...
I think I'd rather have the KOH on it over the Chilli's, I can wash the KOH off easily enough
|
|
|
cnidocyte
Hazard to Others
  
Posts: 214
Registered: 7-7-2010
Member Is Offline
Mood: No Mood
|
|
Very nice. I'm nearly embarrased to show my lab after seeing other peoples labs on sm. I don't have a garage or even a proper shed to build one
unfortunately. I'll get pictures of my lab which is basically a fume hood and a few shelves when I figure out how to hook my phone up to the computer.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cnidocyte  | | Very nice. I'm nearly embarrased to show my lab after seeing other peoples labs on sm. I don't have a garage or even a proper shed to build one
unfortunately. I'll get pictures of my lab which is basically a fume hood and a few shelves when I figure out how to hook my phone up to the computer.
|
Having the computer sat right next to a big open surface with a sink where I'm working is the best.
I only just started doing it, and it makes it so much easier to get better results. Rather than walking away and coming back, I'll spend all day sat
there, browsing the forum, the email, wikis and all that. And I can keep the reaction in sight out of the corner of my eye and stop anything unwanted
as soon as it rears it's head.
I'm using a really old beaten up laptop.
Still works a dream for running the datalogger and checking numbers.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I was searching around google and, again, SM shows up in the results.
But I was scrolling through, getting my gear porn fix for the day, and noticed something.
I have exactly the same Famous Scientists poster that's up on the wall. 
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
I've been silent for too long. Here it goes:
This table sits in my parent's basement in the corner of the shop. The sink is very nice. I didn't have that three years ago.

Pretty standard reagents; nothing too nasty. This is about to change with the coming of my 19/22 distillation kit from DP. Elemental bromine is in the
cards along with hydrazine sulphate, chloroform, conc. nitric acid, and glacial acetic. I have a fume hood but punching a hole in brick when it's two
degrees outside is not fun... it's going to have to wait until spring. In the meantime, I can work in the garage where it's nice and cold.

I think we should all move into one apartment complex and make a SUPERLAB... we could make money synthesizing and shipping quality reagents online to
amateur chemists everywhere. 
Victor Grignard is a methylated spirit.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very nice Doug. The A-1 steak sauce bottle is a nice touch. I also like the Tupperware lid catch pan. All you need now is your fume hood.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I've also had exactly the same pair of scales before.... 
[Edited on 12-12-2010 by peach]
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
@magpie
Yep, I have a bunch of A1 bottles for storing photosensitive things like chloroform. I also love the sauce on just about everything edible. 
Also, the catch pans aren't tupperware but borosilicate glass plates from the inside of microwave ovens I've taken apart for experiments. They're very
sturdy and heat-resistant plates that are excellent for catching H2SO4 before it turns my tabletop to carbon. They'll also take a fair amount of fluid
in the event of a spill and can handle anything my glassware can.
I'm currently getting to truly know the chem department at my school and they told me they'll contact me any time they're getting rid of equipment. I
hope to acquire an analytical balance soon because my AWS scale can only measure up to 100g to .01g. I switched my major from mechanical engineering
to chemistry last semester so I'm fairly sure my home lab is going to start getting awesome in the near future!
-DTM
Victor Grignard is a methylated spirit.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
{edit}Like the vacuum pump posts, I am making this post as an attempt to highlight that not everything that glitters is gold. Laboratory balances that
look scientific are good, but there are very good and usable things in your kitchen. You simply have to know what it can and can't do. In this
investigation.... your wife / mums / gf's fat fighting kitchen scales and cheapo drug dealer pocket balances versus a 3k, calibrated, 40kg lab
balance. Something important to note is that I have had the kitchen scales for over a decade. They've never been checked or calibrated, but they have
had a serious battering - they were in a primary school at one point, enough said. The pocket scales, again, no checking, no calibration, years old,
bits of agar stuck to them and so on. These are not special examples that I've cheated with, you can undoubtedly keep the scales in better condition
than these have been kept as they were never intended for accurate work and have been used by countless people - some of them young enough they're
still learning to write. To check them, all I did was pull them out of the cupboard, stick them on the surface beside the balance and start swapping
masses between them.{/edit}

The red line is for this horrible looking cheapo thing.
The green line is for this Weight Watchers Calorie Counting digital kitchen scale. Not mine.... I swear...

The kitchen scales are on the left axis, the tiny pocket balance is on the right. The x-axis is random weights in grams. They're not entirely random,
they're stacks of coins I put on the big balance, then took off and weighed on the other two.
The first thing that stunned me was how accurate the cheap, 0.01g scale was for that price! Less than 1% error at 0.01g for less than a tenner. The
error is (for most practical intents) always positive as well, so you can predict that the error will give you slightly more material than less.
The next thing that's interesting is that the digital kitchen scale becomes far more accurate at about the end of the smaller one's range. So... the
two together can produce good levels of accuracy when combined. A 100g pocket balance will be even more useful as it'll cover more of the range where
the kitchen scale is off.
The kitchen scale error drops to 1% or less when the mass goes over 100g.
Answer.... use the drug dealer style pocket balance to 50 - 100g. Then switch to the kitchen scale. Don't use the kitchen scale for 3g, unless you're
only after a rough guess. Those two will cost around a hundred times less than a new lab balance like this.
I have also weighed piles of change from a big jar and the accuracies of the coins is, again, remarkably good. It gets even better when multiple coins
are used as one mass; as the errors are periodic, error canceling begins to function, and very well I would say as well.
I have graphs and tables for all these coins, and other methods like DIY balance beams made out rulers and splitting by eye, and will need to upload
them when I get some time.
I'm checking these against this lump. That is the calibration sticker on the side and it's sitting on a granite platform with isolation feet under
that.

You don't need that level of accuracy for most at home work. It only starts getting useful when dealing with micro chemistry. And most people don't
seem too interested in that; e.g. working with 1ml of material. Balances like this are also trickier to use than a lot of people think. They won't
display a stable reading (or any reading at all sometimes) if the balance is upset in anyway; e.g. if it's not been on for 24h, if the temperature in
the room has changed too much, if the air pressure is changing (weather front coming over), if the sample is hydroscopic or the sample or container
are electrostatically charged in anyway, or if there are any convection drafts and so on (it does it even with the door closed). They're also VERY
easy to damage / knock off linear / calibration.
As with everything, the balance is only part of the picture. Trying to reflux / distill / transfer gram quantities or less is not as easy as it
sounds. Transfer it badly, and the accuracy of the balance is essentially worthless. There is also the methodology used in an experiment. For example,
it's not hard at all to change the yield of an experiment by more than a percent (it can go by tens of percentage) based purely on what you've chosen
to use and how you've run it. Knowing the yield to 0.00000000000001% isn't much help if the tolerances on your hands and sense of judgment are off by
30%.

[Edited on 13-12-2010 by peach]
|
|
|
| Pages:
1
..
9
10
11
12
13
..
28 |