| Pages:
1
..
9
10
11
12
13
..
40 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Sodium Chlorate crystals with some Sulphur.

|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Amazing! Beautiful crystals. How did you make them?
Rest In Pieces!
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
They just formed at the bottom of an evaporating Sodium Chlorate solution.
They actually look better with the eye than in the picture. Bad Photography. You could make jewellery with them. Not too good on a wet day though.
I lit the Sulphur that was sitting on the crystal expecting some reaction but funny enough the Sulphur burned itself out without reacting with the
Chlorate and left a thin layer of plastic Sulphur sitting on the crystal.
Sulphur and Sodium Chlorate should not be used together in Pyrotechnics.
Dann2
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I walked into my lab and was greeted with a pleasant surprise. The urea I extracted from fertiliser crystallised into beautiful large crystals.
Picture:

"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Nice! How long did it took to dry?
I never asked for this.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Those are some nice crystals. 
Do you guys ever get into situations where you need the chemical which crystalized with stunning beauty and size, or you need the glassware where it
happened in? What do you do? Do you make another sample, buy more glassware or you destroy the crystals?
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Endimion17  | Those are some nice crystals. 
Do you guys ever get into situations where you need the chemical which crystalized with stunning beauty and size, or you need the glassware where it
happened in? What do you do? Do you make another sample, buy more glassware or you destroy the crystals? |
Break those crystals! Take a pic to post in this thread and then pulverize them.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I can't agree more. That's exactly what I did! Apparently, urea crystals are really fragile, I agitated the jar and all the crystals came loose and
turned into a slush. I can post a picture, but it isn't pretty
To be honest, I was expecting the water to evaporate and leave a mushy amorphous semisolid of urea mixed with water, I wasn't expecting crystals. The
fact I got crystals is quite surprising.
Did anyone else accidentally grow large crystals of urea? The only pictures of urea crystals I found so far were of the microscopic kind.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Approximately a month, but they're not completely dry yet.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
^^nor they'll ever become. Industrial purity urea is hygroscopic, sometimes even deliquescent (lower quality, moist environment). The more times you
try to recristalize it, the less hygroscopic it becomes. Judging by the color of your sample, they aren't pure enough.
But I was thinking about really stunning, large crystals or even monocrystals. I'd probably store them for good unless the substance is for some
reason too important for my work.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Endimion17  | ^^nor they'll ever become. Industrial purity urea is hygroscopic, sometimes even deliquescent (lower quality, moist environment). The more times you
try to recristalize it, the less hygroscopic it becomes. Judging by the color of your sample, they aren't pure enough.
But I was thinking about really stunning, large crystals or even monocrystals. I'd probably store them for good unless the substance is for some
reason too important for my work. |
I know urea is a desiccant, I've actually bought some things that had urea packs to absorb moisture instead of silica gel; sodium hydroxide is not
used for obvious reasons
For this first purification, I wasn't aiming for high purity. I bought urea based fertiliser from Lowes and it contains ~30% urea (highest content of
all the the fertilisers). The rest of it is "slow release polymer coated sulfur" among other things, most of which are insoluble in water.
Boiling fertiliser in water was the simplest way to extract the urea from all the other junk. I boiled it in a soda lime mason jar, the bottom of
which blew off and made a mess. I mopped up all the water solution and put it in another jar to dry, where I forgot it until today. This is why my
sample is impure
I'm wondering if I'd get a higher yield by evaporating urine, considering all the trouble I went through for so little urea.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Unknown copper compound crystallized at the bottom of a beaker:
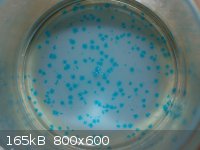

I have larger resolution images that provide a much better view, but I can't find a way around the 800 pixel image width limit.
These were formed from an attempt to neutralize a copper(II) chloride solution with baking soda. Bicarbonate was added to the blue solution until
fizzing stopped, but the blue color remained. I had thought to precipitate copper carbonate, for easier disposal. I left the clear solution overnight,
and came back to see these beautiful tiny crystals! They look like copper(II) chloride still, strangely. They are sitting on a white layer of
unreacted baking soda.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'd have to look it up but that would normally give basic copper chloride: Cu2(OH)3Cl (aka verdigris).
See also this thread here:
http://www.sciencemadness.org/talk/viewthread.php?tid=16811&...
Baking soda would probably just give the right alkalinity for this. With stronger alkali like NaOH adding more leads to the hydroxychloride to be
converted to simple hydoxide (Cu(OH)2)... Baking soda solution is alkaline but doesn't contain much CO3 (2-).
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by White Yeti  | I know urea is a desiccant, I've actually bought some things that had urea packs to absorb moisture instead of silica gel; sodium hydroxide is not
used for obvious reasons
For this first purification, I wasn't aiming for high purity. I bought urea based fertiliser from Lowes and it contains ~30% urea (highest content of
all the the fertilisers). The rest of it is "slow release polymer coated sulfur" among other things, most of which are insoluble in water.
Boiling fertiliser in water was the simplest way to extract the urea from all the other junk. I boiled it in a soda lime mason jar, the bottom of
which blew off and made a mess. I mopped up all the water solution and put it in another jar to dry, where I forgot it until today. This is why my
sample is impure
I'm wondering if I'd get a higher yield by evaporating urine, considering all the trouble I went through for so little urea. |
I'm surprised to hear 30% is the highest content you can buy. I thought technical grade urea is widely available. I can go into a store and buy tons
of it every day.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Endimion17  |
I'm surprised to hear 30% is the highest content you can buy. I thought technical grade urea is widely available. I can go into a store and buy tons
of it every day. |
Maybe I'm looking in the wrong store. I hear lots of people go to Home Depot for chemicals, but I live closer to a Lowes instead. I spent half an hour
looking through all their fertilisers and the highest I could find was 30%.
I also looked for sulfuric acid drain cleaner and copper sulphate, neither of which I could find. I'll make myself a note to check out if Home Depot
has a larger selection of chemicals.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Those urea crystals are really cool looking. They are nice and big.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | Quote: Originally posted by Endimion17  |
I'm surprised to hear 30% is the highest content you can buy. I thought technical grade urea is widely available. I can go into a store and buy tons
of it every day. |
Maybe I'm looking in the wrong store. I hear lots of people go to Home Depot for chemicals, but I live closer to a Lowes instead. I spent half an hour
looking through all their fertilisers and the highest I could find was 30%.
I also looked for sulfuric acid drain cleaner and copper sulphate, neither of which I could find. I'll make myself a note to check out if Home Depot
has a larger selection of chemicals. |
You might be looking at the NPK ratio. Urea is 46% nitrogen.
|
|
|
tastyphenome
Harmless

Posts: 25
Registered: 13-2-2012
Member Is Offline
Mood: No Mood
|
|
Urea can be purchased in high quality dirt cheap from anyone selling tie dye stuff. internet, hippy store, hobby/craft store. soda ash aswell.
edit: Also, best quick fix for sulfuric IMO is autozone/orielleys/advanced auto parts stores. they sell a 6qt and 5gal in my town. not every location
has it in stock. 3/5 will in my area. this is aprox 51% it boils down to very very light discoloration. this can be fixed with H2O2. ask for wet cell
electrolyte. they might just call it battery acid.
12$usd for 6qt@51%=cheap for clean otc. i found much better chemicals once i stopped looking at home depot/lowes. only good for solvents(minus
toluene/dcm/heptane) and sotra cheap roebec lye. in my area atleast.
also hcl. tho slightly discolored/dirty.
[Edited on 16-2-2012 by tastyphenome]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Thanks for the suggestions, but this is not a thread dedicated to buying and finding chemicals. In any case, the recrystallisation worked like a
charm, and strangely enough, the solution did not creep up the sides. Does anyone know why?
Whenever I do recrystallisations of any kind, I always get "creep" where the solution would go up the sides of the container and sometimes go over the
rim.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Quote: Originally posted by White Yeti  | Thanks for the suggestions, but this is not a thread dedicated to buying and finding chemicals. In any case, the recrystallisation worked like a
charm, and strangely enough, the solution did not creep up the sides. Does anyone know why?
Whenever I do recrystallisations of any kind, I always get "creep" where the solution would go up the sides of the container and sometimes go over the
rim. |
I usualy get that creep when i use water as a solvent, so I think it has something to do with the meniscus and the surface tension.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by zoombafu  | | I usualy get that creep when i use water as a solvent, so I think it has something to do with the meniscus and the surface tension.
|
I used water to dissolve the urea, it would be really stupid to use anything else. Cohesion and adhesion forces are definitely part of the story.
Perhaps water does not adhere to solid urea? Thus the crystals formed do not wick up additional solution....
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Here's a picture I took of some copper II chloride crystals that I produced.

|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Interesting...
About CuCl2, I got a colourful equilibrium going between copper acetate and copper chloride once.
I dropped some CaCl2 into some copper acetate solution and swirled. Green a copper chloride solution would form around the CaCl2 and then it would
turn into blue acetate again after some time. If you swirrled it again, the copper chloride would form again, and disappear just as fast. I'll post a
picture if I get the chance.
My camera doesn't bring out the colours very well, but it's a fun thing to try out if you're really bored.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
Quote: Originally posted by White Yeti  | Interesting...
About CuCl2, I got a colourful equilibrium going between copper acetate and copper chloride once.
I dropped some CaCl2 into some copper acetate solution and swirled. Green a copper chloride solution would form around the CaCl2 and then it would
turn into blue acetate again after some time. If you swirrled it again, the copper chloride would form again, and disappear just as fast. I'll post a
picture if I get the chance.
My camera doesn't bring out the colours very well, but it's a fun thing to try out if you're really bored. |
Ill make sure to try that in the future
|
|
|
Vlassis
Harmless

Posts: 9
Registered: 16-10-2011
Location: Athens
Member Is Offline
Mood: Ok.
|
|
Salicylic Acid Needles
[Edited on 1-3-2012 by Vlassis]

[Edited on 1-3-2012 by Vlassis]

|
|
|
| Pages:
1
..
9
10
11
12
13
..
40 |