| Pages:
1
..
99
100
101
102
103
104 |
Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Question
Why does magnesium metal turn black in air?
I first believed that it's because the surface oxidizes. But, both MgO and MgCO3 are white, not black.
And I'm not aware of any black magnesium compounds.
Why is this?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2826
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I think that small particles of unoxidized Mg are incorporated into the tarnish layer, darkening it. Not sure though. Manganese is a common alloying
additive for magnesium, but it's used in extremely small amounts (<1%).
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Would it be a sulphite you are looking at. Silver turnes black (ish).
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
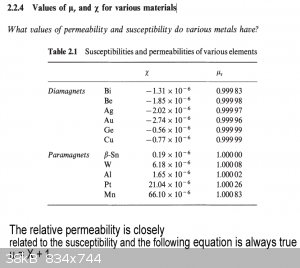
In the book Introduction to magnetism and magnetic materials by David Jiles page 34 it gives a table
shown below. The figures of x and ur do not seem to make sense (according to the formula below the
table).
Am I missing something or is the table wrong?
Yob
|
|
|
khlor
Hazard to Others
  
Posts: 111
Registered: 4-1-2014
Location: Who knows, really...
Member Is Offline
Mood: No Mood
|
|
has anyone dissolved lead or tin in an alkaline aqueous solution like it can be done with zinc? NaOH preferably?
"NOOOOOO!!! The mixture is all WROOOOOOONG!"
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If you want a Sodium Plumbate (I believe it is called) you can dissolve Lead oxide (PbO) in NaOH.
Yob
[Edited on 15-9-2023 by yobbo II]
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
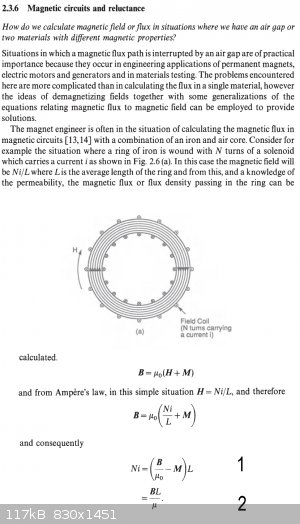
Maths leg up required (get out the spoons)
Can anyone tell me how to get from line 1 to line 2 in the pictures attached.
Thanks,
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
This case is now closed 
B = UoH + UoM
UoM = B - UoH
Divide both sides by H
Uo X = (B/H) - Uo [ X = M/H = susceptance = Ur -1 BTW]
Uo(Ur - 1) = (B/H) - Uo
UoUr - Uo = (B/H) - Uo
B = UoUrH
B = U H
and the second one
B = Uo(H + M)
B = Uo(Ni/L + M) H = Ni/L
B = UoUr(HI/L) FROM RESULT ABOVE
B = U Ni/L
Ni = BL/U
Cheers,
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Hello,
Not too sure if this is appropriate here .. but
I am trying to sort a spread sheet on a certain columb.
The columb in questing contains a very large number, 15 digits.
I do not want to sort the sheet on the actual value of this number but rather on the value of the last three digits of the number.
In other words I want to sort the sheet using the last three digits of the columb containing the very large number.
Cannot figure it out. Using a fairly old version of excell.
If I could AND the number with 0000000000000111 (12 zeroes and three ones) I would strip away the digits I do not want (create a new columb and
sort on the new columb but I am unable to do this AND.
Cheers,
Yob
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by yobbo II  |
Hello,
Not too sure if this is appropriate here .. but
I am trying to sort a spread sheet on a certain columb.
The columb in questing contains a very large number, 15 digits.
I do not want to sort the sheet on the actual value of this number but rather on the value of the last three digits of the number.
In other words I want to sort the sheet using the last three digits of the columb containing the very large number.
Cannot figure it out. Using a fairly old version of excell.
If I could AND the number with 0000000000000111 (12 zeroes and three ones) I would strip away the digits I do not want (create a new columb and
sort on the new columb but I am unable to do this AND.
Cheers,
Yob |
Insert a new column.
In the first row of your new column include the formula
=RIGHT(A1,3)
assuming your large number is in column A starting in row one, so the first number you want to sort is in cell A1.
Drag the formula to the bottom of the sheet.
Select all of the columns that you want to sort and sort by your new column.
Is this what you were trying to achieve?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....i have 2-tert butyl-1-phenyl ethanol and i want to nitrate the ring, i was thinking of using anhydrous ethanol as my solvent since the material is
a powder, or can someone suggest another solvent that i can use.....solo
[Edited on 18-10-2023 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Thanks alot B(a)P,
That was exactly what I needed.
There is still a small problem but only a small one. I need to do the sorting very seldom.
It may be just a software glitch from using old Works.
When I paste in my info. the column (col A) with the large number gets pasted in in scientific notation (the number in notepad before pasting is just
a simple 15 digit number).
I convert the column (A) to text and the number stayes as scientific notation.
The only way to 'convert' back to a 15 digit number is to double click on the number.
Sounds a bit weird.
Any suggestions?
The picture explaines things a bit better.
Thanks alot,
Yob
@solo appologies for talking over you!

[Edited on 17-10-2023 by yobbo II]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by yobbo II  | Thanks alot B(a)P,
When I paste in my info. the column (col A) with the large number gets pasted in in scientific notation (the number in notepad before pasting is just
a simple 15 digit number).
I convert the column (A) to text and the number stayes as scientific notation.
The only way to 'convert' back to a 15 digit number is to double click on the number.
Sounds a bit weird.
Any suggestions?
[Edited on 17-10-2023 by yobbo II] |
Make the column wide enough to accomodate 15 digits. Select the column, then format as a number and it should go back to non-scientific notation. The
default format for numbers is two decimal places so you may need to fix that as well by pressing the decrease decimal button a couple of times.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2816
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Or just use the MOD function and do X MOD 1000, which will return only the remainder of a division by 1000. For the text to work, you would need to
use right(text(X,0),3) or something like that to convert the number to text, then use the right 3 digits. You can also use the format cell feature
to set the cells to "text", where numbers are not converted to a real value, but saved as the text characters, and then right(x,3) will work.
|
|
|
yobbo II
National Hazard
   
Posts: 773
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
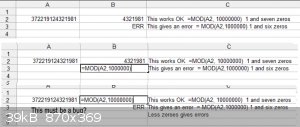
I cannot get the =MOD(Ax, 1xxx) to work as the sheet gives an error. This must be a bug?
Both B2 and B3 are formatted as numbers. Setting greater number of decimal places does not help (It should not matter anyways)
See the picture
I got the sheet to work with the following:
I used the =string(Ax,0) (same as =text(xxx) I guess) to convert to general format this gets rid of the scientific notation
followed by =right(Ax, 4) to give me the four digits to sort on.
Thanks for you time
|
|
|
Dr.Bob
International Hazard
    
Posts: 2816
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
I just checked and it seemed to work fine for me:
=MOD(2387373278378,1000) provides 378 for me.
but if the text way worked, that is fine as well.
|
|
|
Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Can copper sulfide be made by electrolysis sodium sulfide solution using copper anode? will the rest of the solution be just sodium hydroxide?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I wouldn't expect so- I'd guess that the anode would become coated with non-conductive copper(II) sulphide. If you had any other copper salt in
solution or suspension, it should react with the sodium sulphide solution to give CuS, which is ridiculously insoluble.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
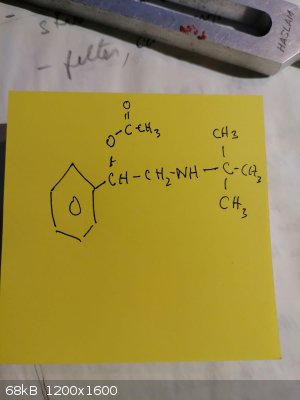
...help finding the correct name for compound ...i tried 2-ethyltert butylamine-1-phenylbenzoate ...but no results....its a steroid precursor
.....solo
[Edited on 4-11-2023 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
2-(tert-butylamino)-1-phenylethyl acetate
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
...thanks Bedlasky any suggestions on the type of solvent to use in a p-nitration of this 2-(tert-butylamino)-1-phenylethyl acetate or its
2-(tert-butylamino)-1-phenylethanol alcohol.....solo
note: I have searched around and found two candidatea, methanol and DCM
[Edited on 12-11-2023 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
FableP
Harmless

Posts: 10
Registered: 26-7-2023
Member Is Offline
Mood: No Mood
|
|
Likely a very noob question here, I have seen a synthesis for hydroxylamine HCL where nitromethane and HCl are refluxed for many hours, similarly I've
seen the same for hydroxylamine sulphate with nitromethane and sulphuric acid, however when looking on how to synthesise hydroxylamine nitrate, most
synthesis point towards reacting barium nitrate with hydroxylamine sulphate.
Will the following proceed to hydroxylamine nitrate: CH3NO2 + HNO3 + H2O → [NH3OH]NO3 + HCOOH
or is some other reaction pathway followed for a different product?
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I don't think that this will work. Hydroxylamine is strong reducing agent and refluxing it with HNO3 will probably destroy it. It could be actually
pretty dangerous, because hydroxylamine reacts with some oxidizing agents pretty violently even in aqueous solution:
https://woelen.homescience.net/science/chem/exps/hydroxylami...
I hope you know that hydroxylammonium nitrate is explosive.
|
|
|
FableP
Harmless

Posts: 10
Registered: 26-7-2023
Member Is Offline
Mood: No Mood
|
|
I'm planning a HiPEP project. It looks like BaNO3 might be the way to go.
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
HiPEP??
I have no idea if you are taking about a herbal remedy claimed to cure intestinal bloating or High Power Electric Propulsion.
Please make your posts clear.
In the meantime, the formula for barium nitrate is Ba(NO3)2.
|
|
|
| Pages:
1
..
99
100
101
102
103
104 |