| Pages:
1
..
8
9
10 |
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I did a few exploratory experiments with these carbon foams and a sodium silicate solution (waterglass). I tried three basic treatments:
* repeatedly soaking in waterglass, then dilute sulfuric acid, then rinsing
(Na2O)x·(SiO2)y + xH2SO4 ->
xH2O + ySiO2 + xNa2SO4
the idea was to coat or permeate the foam with silica. The foam was baked to dry after each dunking in the waterglass, which led to the formation of
many tiny sodium silicate bubbles. These structures survive the acid treatment as this photomicrograph show. At high magnification, there appear to be
small silicate needles on the carbon.
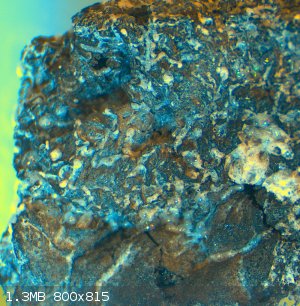 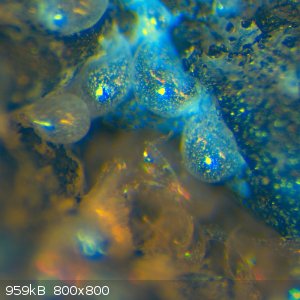
It seemed to stand up to a MAPP gas torch pretty well; afterwards, the pores of the carbon foam seemed to have been filled with a bubbly/crystalline
silicate

* soaking in boric acid, baking to dehydrate to boric trioxide, then soaking in waterglass
(Na2O)x·(SiO2)y + zB2O3 ->
(Na2O)x·(SiO2)y·zB2O3
Boric acid was dissolved in absolute EtOH, and the carbon foam soaked in the solution. It was dried, then baked to dehydrate, then soaked again. After
a couple of repetitions, this was dunked in waterglass and baked dry again. The result was a fluffy, flakey white crust on the foam. When hit with the
MAPP gas, the whole structure crumpled, suggesting that the boric/silicate soak had permeated and disrupted the carbon network, such that it lost all
integrity when the intersitial material melted. When examined, the silicate/boric material had melted into droplets clinging to the foam surface,
which cooled into tiny beads containing air bubbles. I wonder what role the foam geometry (voids, ridges) have in forming the beads?
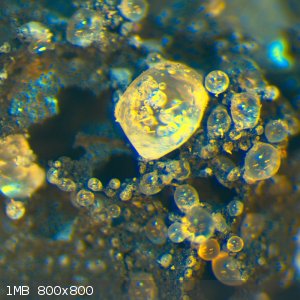
* soaking in copper acetate, then soaking in waterglass, then dilute sulfuric
The idea here was to coat the foam with a mixed copper/silicate layer, like you'd see in a chemical garden. After soaking in an ethanol/copper acetate
solution and drying, a chunk of foam was cut open. The salt appeared to be mostly but not entirely superficial, with a small amount appearing to have
made its way into the interior. When hit with a torch, a thin layer of copper was left firmly adhering to the surface.
After soaking in waterglass, drying, and then sealing with dilute acid, the surface had a light green, foamy appearance. When this was torched, it
seems to withstand a lot of heat; afterwards there is a glassy red-brown residue on the surface. Under the microscope it looks to be a mix of copper
metal and silicate. It also looks like the silicate is wetting the carbon surface much better in this case than the others.
I wonder to what extent the cupric ion is being reduced by the acetate ion vs. by the carbon substrate...
 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Stein, B. E., Auciello, O., Arellano-Jimenez, M. J., & Perez, B. R. (2022). Fungal Mycelium Conversion into Ultrananocrystalline Diamond via
Microwave Plasma Pyrolysis. ACS Sustainable Chemistry & Engineering, 10(10), 3211–3218. https://doi.org/10.1021/acssuschemeng.1c07253
| Quote: |
ABSTRACT: Fungal mycelium has been touted as an environ- mentally sustainable potential replacement for a wide range of materials commonly used today,
including textiles, building materials, and medical bandages. Additional applications of mycelium are possible through pyrolysis, wherein chitin in
the mycelium is carbonized to produce useful carbon allotropes like biochar or activated carbon. Here, we demonstrate that this pyrolysis
process can be achieved quickly and efficiently with a plasma reactor built around a commonly available kitchen microwave oven. It is found
here that this microwave plasma pyrolysis (MPP) process converts the mycelium into a “myco- diamond” matrix containing
ultrananocrystalline diamond (UNCD) nanostructures with macro-, micro-, and nanoscale features. Verification of the MPP-induced
transformation of mycelium into UNCD is confirmed via complementary material analysis techniques including Raman spectroscopy, high-resolution
transmission electron microscopy, and X-ray diffraction. Analysis of the myco-diamond surface morphology was performed via scanning electron
microscopy. The MPP process represents a scalable and low-cost method of producing UNCD nanomaterials derived from renewable biological sources.
|
Attachment: Fungal Mycelium Conversion into Ultrananocrystalline Diamond via Microwave Plasma Pyrolysis-compressed.pdf (1.8MB)
This file has been downloaded 363 times
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
| Pages:
1
..
8
9
10 |
|