| Pages:
1
..
8
9
10 |
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I also made the barium salt of borotungstic acid by adding the acid to BaCO3. Ba5[BW12O40]2
weighing in at 6399.3g/mol, I just never left time for it to crystallize. I suspected someone might have a higher compound waiting so I waited.
[Edited on 12-15-2014 by gdflp]
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Molybdenum what?
Compound name: Molybdenum blue
Molecular weight: No idea! Lots though.
Materials:
-Sodium molybdate
-Concentrated Glucose/Fructose solution, prepared by dissolving 200g of -sucrose in 350ml of water, adding a few drops of 30% HCl, then heating.
-30% HCl
Synthesis: ~5g of sodium molybdate was dissolved in ~15ml of the concentrated glucose/fructose solution. This took about 10 minutes, with
swirling. ~10ml of 30% HCl solution was then added and the mixture was left to stand for several weeks, suring which time a very dark
precipitate formed of the unknown molybdenum complex.
This mixture was then filtered:

and dried:

to obtain a yield of 1.41g (I don't trust the last decimal place on that scale.).

Discussion:
Although this is clearly some kind of molybdenum blue complex, its precise nature is unclear. Glucose and Fructose are reducing agents because they
have an equilibrium with an open ring structure, at one end of which is an aldehyde and so I surmised that they might be able to Create a molybdenum
blue complex, since many other reducing agents can including:
Hydrazine sulfate
Sodium dithionite
Sodium metabisulphite
Sodium thiosulphate
Sodium hypophosphite
And a whole bunch of others (see attached paper)
However it is unclear which compound I have formed, since all of the characterised compounds in the attached paper have some kind of cation (either
sodium or ammonium) and so I think that the actual molybdate complex is probably present as an anion. However I don't see how this can be the case
with my compound, since I can't see anything that could form a cation. I think perhaps I may have synthesised a complex that is not composed of
cations and anions, which would explain its low solubility in water.
Finally I'd just like to say that I don't expect this to be accepted as a valid entry, but I hope that some other members could shed some light on the
possible nature of this compound.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Didn't you use sodium molybdate? If so, couldn't the sodium be the cation? My guess is that it is some sort of compound with a sodium cation, and an
organomolybdenum complexed anion. In any event it's a rather interesting synthesis and that's a very neat color.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
I found that my borax was able to reduce molybdate to molybdenum blue when i was trying to make boromolybdates.
Mailinmypocket, now you need to write the balanced equation 
Just kidding
[Edited on 15-12-2014 by bismuthate]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Congratulations gdflp for winning the challenge!
You win $27 USD.
Please U2U me to verify that sending the money to your PayPal account is the collection method of choice.
Thank you to everyone who participated in the challenge, especially if you donated. There are a lot of great synthesis reports in this thread, and I
look forward to seeing more in upcoming challenges.
Great work, everybody!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
so i didn't win then ?
damn.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Come away, son, Dad will buy you an ice cream...
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Lol,
Nice job everyone. There was a lot of activity in the end, too bad Blogfast missed the entry-time.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bismuthate  | I found that my borax was able to reduce molybdate to molybdenum blue when i was trying to make boromolybdates.
Mailinmypocket, now you need to write the balanced equation 
Just kidding
[Edited on 15-12-2014 by bismuthate] |
This time of year I can't even balance my diet 
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Congratz to everyone who submitted an entry and gdflp for winning!
I'm disappointed nobody found this gem... the crystalline 77888 amu
H2496Mo368Na48O2464S48 molyblue (structure confirmed by single crystal x-ray diff.). I'm
posting it below for anyone who wants to synthesise a wopper 
Full text with impressive crystal structure available for free here:
http://www.researchgate.net/publication/10983409_Inorganic_c...
This paper was "Dedicated to Professor Dieter Fenske
on the occasion of his 60th birthday". 
Structure of the beast from [1]:
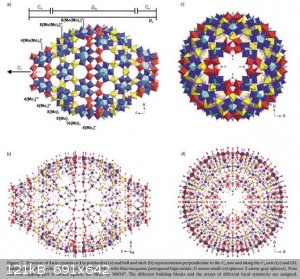
Synthesis [1]:
| Quote: | Na2S2O4 (0.15 g, 0.86 mmol) as reducing agent was added to a stirred solution of Na2MoO4.2H2O (3 g, 12.4 mmol) in water (10 mL) which was acidified
with 0.5� H2SO4 (35 mL; immediate color change to blue). The solution was stored in a closed flask and after 2 weeks the precipitated deep-blue
crystals of 1 were collected by filtration, yield: 80 mg (two crystal types: major part showing from top view a characteristic form like an elongated
benzene molecule, while a much smaller fraction of the crystals is grown together).
Characteristic IR bands (KBr pellet): � � 1616 (m,(H2O)), 1191 (w), 1122 (w), 1060 (all �as(SO4)), 975/954 (s) (�(Mo�O)), 761 (s), 700 (sh), 627 (w),
555 (m), 464 (w) cm�1; approximate wavenumbers of the very broad Raman bands (KBr dilution; �e � 1064nm): � � 810 (m), 680 (w), 460 (m) cm�1; Vis (in
H2O): � � 740 (very broad) nm. |
Reference:
[1] Achim Müller et al. Inorganic Chemistry Goes Protein Size: A Mo368 Nano-Hedgehog Initiating Nanochemistry by Symmetry
Breaking. Angewandte Chemie International Edition. 2002, 114, Nr. 7, 1210-1215.
[Edited on 18-12-2014 by deltaH]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
!!!
Is each molecule the size of a small car ?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I heard a polio virus tried to hump it 
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
You know, there's just been nothing worth responding to on SM today...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No, I didn't enter it, in a spirit of protest. Think of that what you will.
But it sure was entertaining enough... 
Maybe next time...
[Edited on 18-12-2014 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Na2S2O4 (0.15 g, 0.86 mmol) as reducing agent was added to a stirred solution of Na2MoO4.2H2O (3 g, 12.4 mmol) in water (10 mL) which was acidified
with 0.5� H2SO4 (35 mL; immediate color change to blue). The solution was stored in a closed flask and after 2 weeks the precipitated deep-blue
crystals of 1 were collected by filtration, yield: 80 mg (two crystal types: major part showing from top view a characteristic form like an elongated
benzene molecule, while a much smaller fraction of the crystals is grown together). |
That's a really interesting synthesis! Not having sodium dithionite, I found (Wikipedia) it can be made from the reaction between sodium bisulfite and
zinc: 2 NaHSO3 + Zn → Na2S2O4 + Zn(OH)2
I don't suppose sodium metabisulfite would work as a replacement? That's what I have on hand at the moment.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | That's a really interesting synthesis! Not having sodium dithionite, I found (Wikipedia) it can be made from the reaction between sodium bisulfite and
zinc: 2 NaHSO3 + Zn → Na2S2O4 + Zn(OH)2
I don't suppose sodium metabisulfite would work as a replacement? That's what I have on hand at the moment. |
Yes, most sulphites (OS of S < 6) should work. I got that blue with ordinary sodium sulphite. There's also a recipe with a hypophosphite. Just
adjust for t'electrons. 
The reduction really doesn't seem very dependent on the type of reducing agent. I'm guessing even plain zinc might work.
[Edited on 18-12-2014 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I was also thinking of metabisulfite as it's easily available, but I'm guessing there's a good reason to use the dithionite... though exactly why
eludes me. I suppose one could always email the authors about it. This is an incredibly impressive example of synthetic inorganic chemistry, so I
doubt the authors overlooked the use of less exotic reagents.
This molyblue has sulfate units as part of its structure, so the neat thing about the reducing agent is that it is oxidised to sulfate... so no
contaminant is introduced. Perhaps it's important to have only the reduced moly salts and sulfate in solution for the crystals to form.
Anyhow, if you get crystals after two weeks with a "characteristic form like an elongated benzene molecule"... I'd say you can safely assume success.

[Edited on 18-12-2014 by deltaH]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
To be clear, I meant using metabisulfite as a substitute for bisulfite in the equation I posted. I still planned on using dithionite for the actual
moly blue reaction. The plan would be as follows:
1) Make a very cold solution of sodium metabisulfite and stir in zinc powder.
2) Filter off unreacted zinc and the zinc hydroxide precipitate. The solution now contains dithionite (Soln. A)
3) Make a solution of sodium molybdate (or ammonium molydbate, can't remember which I have) acidified with sulfuric acid (Soln. B).
4) Combine solutions A and B with stirring. After mixing, cap and set aside for several weeks.
5) Profit.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Ah ok, I misunderstood you. Best to follow a literature recipe for the dithionite... since it's for profit 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
On what grounds though? A reduction is a reduction, in most cases.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Experience tells that even the simplest chemistry sometimes doesn't work or performs poorly when not done under specific conditions... it doesn't hurt
to check the literature when it's available.
Perhaps there is a competing reaction that can be minimised, for example, just looking at that equation above, the reaction
Zn + HSO3(-) => Zn(+) + SO3(2-) + 1/2H2
springs to mind as a possible problem, haven't checked reduction potentials though.
Anyhow, whatever the case be... a cursory check of the literature would not hurt when it's probably available.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deltaH  |
I'm disappointed nobody found this gem... the crystalline 77888 amu
H2496Mo368Na48O2464S48 molyblue (structure confirmed by single crystal x-ray diff.). I'm
posting it below for anyone who wants to synthesise a wopper 
|
Interesting. In the paper I attached there is a synthesis exactly the same as the one you posted, except they used HCl as opposed to H2SO4. They get
an entirely different product, however.
I wonder how the different acids could have affected the compound formed? Or is one (or both) of the papers incorrect in their analysis...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The difference is that the sulfate is part of the structure here where it's not with HCl, AFAIK.
Perhaps the use of other heteropoly acid forming inorganic acid units could also lead to super giant structures, for example using sodium
hypophosphite as the reducing agent and acidifying with phosphoric acid. Sadly NaPO2H2 is classed as a DEA list 1 reagent 
|
|
|
| Pages:
1
..
8
9
10 |