| Pages:
1
..
8
9
10
11
12
13 |
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Thanks then. How do I purify it? Most comments/ threads about purification are based on TNT, not MNT
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I noticed this a while ago in the prepublications section. It should give you some ideas for how to separate MNT isomers from one another.
http://www.sciencemadness.org/talk/viewthread.php?tid=29111
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Hey guys, uh I'm doing some reading into TNT/ nitro - toluene's carcinogenic properties, and the risk/ payoff ratio is sketch to me.... How do you
dudes see it?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Pure alpha TNT apparently has quite low toxicity, however, DNT is apparently very carcinogenic. I just use common sense; limit contact and wash well
before eating or using the bathroom, etc.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Yeah but dude: A problem is DNT vapour as well. 2, 4 - Dinitrotoluene vapour is released (By DNT or TNT I don't know), and the dudes use it to locate
TNT, buried or concealed. That should indicate that there is more than trace vapour, and potential alpha TNT decomposition among even professional/
industrially prepared samples.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
If you have prepared any nitrotoluenes, it is pretty obvious that mono- and dinitrotoluenes have a relatively high vapour pressure, since they have a
very distinctive scent. However, many things that you are exposed to every day are quite carcinogenic (diesel fumes, cigarette smoke, etc.). The
poison is in the dose (and, in the case of carcinogenic substances, especially in repeated exposure). I'd say that unless you plan on making TNT
regularly over a period of several years, you don't really need to take other precautions than what Hennig does.
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Okay so after another attempt at o - Mononitrotoluene, it failed again. Toluene was added to a Nitric/ Sulfuric mixture. What tended to happen was a
red liquid would form, dissolve, and precipitate as a beige solid. The temperature would start low and rise to 50 degrees Celcius. Usually I would
think it to be obvious 2, 4 Dinitrotoluene, but the reaction temperature seemed a bit too low. This is great
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you're nitrating at 50 C, a lot of DNT will be formed. If you can keep it below 20 C, then you will only get a few percent DNT, especially if you
add the nitric acid to a mixture of the toluene and the sulfuric acid, instead of adding the toluene to the mixed acids (so that nitric acid is never
in excess).
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
How can I separate Para MNT from 2, 4 DNT? As well, the stuff is all heavier than water, a waxy liquid, so I think there's some Otho MNT chilling in
there too. What a bloody mess. Is there anything I can do?
|
|
|
Trotsky
Hazard to Others
  
Posts: 166
Registered: 6-2-2013
Location: US
Member Is Offline
Mood: No Mood
|
|
Should be solid at room temp?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Here is document of interest. It has a graph showing critical diameter of cast Pentolite as a function of percentage PETN.
Attachment: Critical Diameter and Spin Effects in Detonation of Cast and Liquid Explosives.pdf (369kB)
This file has been downloaded 823 times
[Edited on 16-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
DNT step Attempt
So finally i got around to my attempt at synthesizing some TNT-well DNT actually, havent done the DNT to TNT nitration yet but that will be done as
soon as my sodium sulphite order arrives.
So for this synthesis i used 38.5g ammonium nitrate and some %98 tech grade sulfuric acid 85ml, and 11mls dist water,I also acquired some pure
toluene,At least the label says that and its density measurement is pretty spot on a density chart i have, its not "diggers" brand. 25mls of this was
used.
I followed the a synthesis that was done by fellow SM user Hennig Brand a few pages back for a guideline, mostly because he had success with the
method and got some fine looking end product from it although i did scale it down by half.Also i added the toluene to the mixture not vice versa like
HB did. The nitration mixture was made the day before and put in the freezer overnight and warmed to 5c before adding any toluene which was done from
a glass syringe in small portions at a time The whole process ran pretty smoothly with the temp rising slightly with each addition and upon finishing
it was at around 50C. additions were completed fairly fast in 45mins. 15 mins after the last addition of toluene the heating was then applied very
gently with a water bath to 80C where it was held for 1hour 45mins. the mixture was then allowed to cool and the DNT solidified gradually with the
waxy oily looking cake fished out crushed a bit and washed several times with cold water. upon drowning the spent nitration mix a bit more DNT was
recovered also. these were combined and washed again but molten in hot water and agitated for 10 mins and set to cool with the DNT cake solidified in
the vessel. It's still yet to weigh it but the yeild loos good.
The pic of the darker layer im not 100% sure of time in the synthesis but the lighter layered picture was just before i stopped heating it.
The smell of this synthesis was not as bad as i expected either. It kind of took me back to being a kid and being made to get out the tin of nugget
and polish up dads army boots.....
Next step will be the TNT nitration step, and i have enough distilled nitric in the high 90%s for this but it is slightly orange and I would like to
know if this is ok for a TNT nitration? or does it need to be cleaned first? looking forward to completing this project in the next few weeks since i
have been looking at doing this synthesis for quite a while now.NP
  
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Looks good. The one thing I did notice was that you didn't use any excess of nitrate/nitric acid if my calculations are correct. Assuming the AN used
was completely dry and 100% pure, which it wouldn't be, the quantities you gave would give 2% excess of nitrate/nitric acid. I think 10 to 20% excess
would be better to ensure a more complete reaction. You probably used my quantities, but didn't see where I multiplied by 1.1 to give a 10% excess.
Admittedly my post was a little bit hard to follow, and I half intended to go back and make it more streamlined and easy to follow, and post it again,
but never got around to it. A little lower yield and more MNT contamination is not a big problem at this stage anyway.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by Hennig Brand  | | Looks good. The one thing I did notice was that you didn't use any excess of nitrate/nitric acid if my calculations are correct. Assuming the AN used
was completely dry and 100% pure, which it wouldn't be, the quantities you gave would give 2% excess of nitrate/nitric acid. I think 10 to 20% excess
would be better to ensure a more complete reaction. You probably used my quantities, but didn't see where I multiplied by 1.1 to give a 10% excess.
Admittedly my post was a little bit hard to follow, and I half intended to go back and make it more streamlined and easy to follow, and post it again,
but never got around to it. A little lower yield and more MNT contamination is not a big problem at this stage anyway. |
It would be useful to have a bit more acid excess. From experience, the best DNT results come from putting MNT isomer(s) contaminated DNT slag
through a second nitration to completely synth DNT, and recrystallizing it from Methanol. VERY pure DNT is created.
[Edited on 23-3-2015 by Hawkguy]
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
So the yield ended up being 30.8g and it is seemingly contaminated with at least some MNT judging by the smell and after drying there is an oily feel
left on the glove after touching some of the cake. but i guess you learn things by experimenting so im not totally bothered by the product or the
weight of it since this is my first attempt at nitrating toluene. there is obviously room for improvement here and i really just wanted to get a feel
for the process and will be trying again when i get a chance. I will be nitrating the DNT/MNT sample further to see what the end product will be like
and work on improvements from there. if i use excess nitric and extend the heating time i should end up with an OK end product after
washing/purification/recrystallizing with at least some TNT. Working on a Melting point apparatus of some sort may be a good idea and helpful in
determining the TNT content of the final sample.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hawkguy  |
It would be useful to have a bit more acid excess. From experience, the best DNT results come from putting MNT isomer(s) contaminated DNT slag
through a second nitration to completely synth DNT, and recrystallizing it from Methanol. VERY pure DNT is created.
[Edited on 23-3-2015 by Hawkguy] |
What is "best" may not always be the same. On an industrial scale where extremely large quantities are made improving yield by even 1% is a really
big deal. One percent of a 100g batch is only 1g, but 1% of 100 tonnes is 1 tonne and of course economics is the main factor for the people making
tonne quantities whereas for the hobbyist economics is normally a small factor.
I have an extreme sensitivity to MNT, even a couple good whiffs of it will cause the glands in my neck to swell, my throat to get really soar and I
just feel awful. It is easy to run the toluene to DNT nitration so that the product produced smells only slightly of MNT and causes me very little
discomfort. Also, with a little care the nitration of toluene to MNT can be done quite efficiently. When producing MNT, it can also be very difficult
to completely get the smell of MNT off of the equipment that was used, which can be an annoyance for a long time after the synthesis. Also DNT is more
useful to me than MNT, so I am producing a more useful intermediate in one step on the way to TNT. The extra handling, especially since MNT is so
unpleasant to deal with, just isn't worth the slight economic benefits for me for a number of reasons.
For many uses of DNT, small amounts of contaminants including various other nitrotoluenes is not a big deal. A small amount of MNT going into the
final stage of nitration to produce TNT shouldn't be much of a problem either, unless it is a large amount and then the amount of nitration mixture
may need to be increased and the nitration may need to be run a little more carefully at least in the early stages.
[Edited on 23-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
In the middle paragraph of the last post I wrote, "Also, with a little care the nitration of toluene to MNT can be done quite efficiently." It should
have said, "Also, with a little care the [single step] nitration of toluene to DNT can be done quite efficiently."
The following quote and table were taken from the "Detonator Casings" thread. I thought it would be good to include it here.
Quote: Originally posted by Hennig Brand  |
I don't want to get too far off topic, but I thought I would include the following jpg of a table taken from an old defense document (Powder and
Explosives by A. G. Gorst). It provides some minimum mercury fulminate initiator charge weights for cast and pressed TNT and Picric Acid. As you know,
granular TNT and picric acid are much easier to initiate than their cast forms. Very commonly, granular or crystalline TNT or picric acid was used as
a booster to initiate cast TNT and cast picric acid.
BTW, did you check the melting point of your TNT? TNT even when of high purity is relatively insensitive to initiation.
TNT does take a bit of time to make, but after doing it a couple of times I find it fairly straightforward and with the right equipment (large flasks,
etc) it could be made in large quantities relatively safely. It wouldn't take much longer to make a pound batch than it would to make a 15g batch.
|
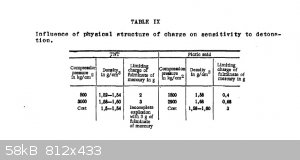
[Edited on 26-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Here is another table, taken from the same text, showing limiting charge weights required to initiate TNT, picric acid and tetryl pressed with the
same loading pressure in copper blasting cap casings. The difference in limiting primary charge weight, required for initiation, between TNT and
picric acid is much smaller than between the two when pressed to practical densities or when cast.
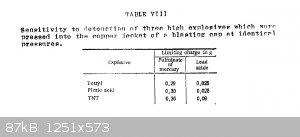
[Edited on 26-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Correct disposal of "red water"
So I completed a TNT synthesis a couple of days ago and got a fairly good result. I won't go into the particulars of the process too much as it has
been discussed quite a lot already on this thread. I'm Now up to the washing/purification stage and will be doing this with a 5-10% hot sulfate
solution. The Part that bothers me is a the Environmentally toxic waste so called "red water" containing the impurities and undesirable isomers. For
obvious reasons we can't just take this waste to a treatment plant or waste disposal facility and its not really something you would want to store
either and since it is pretty much toxic waste flushing it down the drain is out of the question, so what do we do with it? Is there a safe way to
destroy the by products and make the solution safe for disposal down the drain? What have you guys done with this leftover garbage? In the past I just
watered my garden with the spent nitration acids heavily diluted since plants like nitrates and sulfates.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I tried using a sulfite wash a couple of times in the past and it was not convenient at all. It actually damaged my product too which I think had a
lot to do with the temperature being too high. I found recrystallization from either methanol or ethanol to be much less messy and much harder to do
improperly.
There are a lot of articles available giving procedures for how red water can be dealt with, but I never looked into it much because the quantities of
waste I produced have been so tiny. I think industrially it has been a big problem and I think most of the options available are less than ideal,
either not very effective or very costly or both. Apart from finding a chemical additive which could render the waste less of an environmental hazard
I would suggest waiting until you had a good hot campfire or bonfire with lots of hot coals and incinerating it.
[Edited on 5-4-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Attached are a few patents describing some of the issues and solutions related to TNT purification. I should have read these before trying to perform
a sulfite wash earlier on.
Attachment: US3043885.pdf (585kB)
This file has been downloaded 976 times
Attachment: US1975598.pdf (420kB)
This file has been downloaded 763 times
Attachment: US2126162.pdf (329kB)
This file has been downloaded 667 times
Attachment: US2132845.pdf (251kB)
This file has been downloaded 563 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I recall a process using a toluene extraction to salvage the organics that would preferentially dissolved into the toluene layer to clean up the TNT
wash water, then recycleing the toluene & various impurities it contained right back into the next nitro toluenene synthesis. Also mention of
harvesting the various TNT isomers/impurities for other processes and desirable explosive products in COPAE, as they represented a loss of fixed
Nitrogen & inefficiency if wasted.
It may take me a bit to run down the references.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
TNT? DNT?
I just dug up a two year old batch of TNT/DNT that was made using Jared Ledgers crapbook method.
I have the notes written down and I used 98% sulphuric acid and 70% nitric for all the steps. I have the amounts I used somewhere but I cannot find
them at the moment. They were a scale-down from Legard's amounts for the three step nitration in Prep manual of explosives.
For the mononitro step, the nitrating acids were heated to about 43-45 degrees and the toluene added drop-wise from an addition funnel. The mix was
then stirred for 20 minutes at about 50 Degrees before separating the layers.
For the second dinitro step fresh acids were heated to 75 Degrees and the MNT dripped in from an addition funnel again. Addition took about 20
minutes. After the addition , the mix was heated to about 85 degrees for 10 minutes and taken off heat with continued stirring and then put in a sep
funnel and the top layer stored.
For the final trinitro step, the fresh nitrating mix was heated to about 90-92 Degrees and the temperature maintained while the DNT was added.
Addition took about 20 minutes from what i remember. The DNT had to be warmed and melted back down as most of it had crystallized between steps.
After this, the mix was kept at 90 Degrees for 5 minutes and then brought up to 112 Degrees for a further 5 minutes before cooling for 5 minutes and
crashing into ice-water.
The final product has been bicarb washed and water washed under hot 80 Degrees water about 10 times before being bagged and stored.
Finally, the question, without the fuming sulfuric acid (oleum) do you think I would have got any TNT product at all or could it all be DNT? Is it
possible to obtain trinitrotoluene without it?
I will do a melt test tomorrow and see what temperature the product melts at which should give me a fair idea.
[Edited on 30-10-2015 by greenlight]

Be good, otherwise be good at it 
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
I think the heating period for the last step is hours not 5 mins and up to 130c in temperature.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thanks NP, i will do a melt test anyway to get an idea of the dinitrotoluene content.
After the addition of the DNT, Ledgards total heating time is 15 minutes in his book
Looks like i will have to melt it and use a fresh nitration mix with longer time and higher heat to get the third nitro group on. Not a fan of this
method as it uses a lot of sulphuric acid.
[Edited on 1-11-2015 by greenlight]
Be good, otherwise be good at it 
|
|
|
| Pages:
1
..
8
9
10
11
12
13 |