h0lx
Hazard to Self
 
Posts: 69
Registered: 31-12-2005
Member Is Offline
|
|
An Idea
I came across this in bed last night thinking of chemistry(geeky, I know)
What if one would take citric acid and reduce it with a strong reducting agent like LiAlH4 to yield something I would call(sorry for errors)
1,3,5-hydroxy 3-hydroxymethylpentane and then nitrate it to get 1,3,5-nitro 3-nitropentane. This would be probably similiar to PETN in properties.
I need to go through all my chem suppliers to see if they have LiAlH4, I would try it out.
EDIT: Made a crappy paint drawing of it.
[Edited on 4-5-2007 by h0lx]
EDIT2: New better image, thank you Ramiel.

[Edited on 5-5-2007 by h0lx]
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
The middle molecule looks like pentaerythritol.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I don't think that tertiary nitro ester looks very stable.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Looks viable. Only a couple additional methylene groups when compared with PETN. Be sure to look up some standard procedures for reducing carboxylic
acids to alcohols, would be a shame to waste LiAlH4.
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Lithium Aluminum Hydride
Also be aware that LiAlH4 is a watched chemical on the DEA's drug precursor list
if you live in the U.S. any quite probably most places in the world. It may not be worth
the hassle.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
That would work..Except... You would probably be nitrating hydroxyl aconitic acid analogue; the tertiary hydroxyl is prone to dehydrate...
Aconitic acid is the same molecule minus the 3° hydroxyl plus a double bond in either the cis or trans position (trans predominates under
thermodynamic control).
Hmm. Run it cold and slow and it might be do-able (at least with some yield), but that hydroxyl will likely dehydrate if you look at it wrong.
hmm,
O3
[Edited on 5-5-2007 by Ozone]
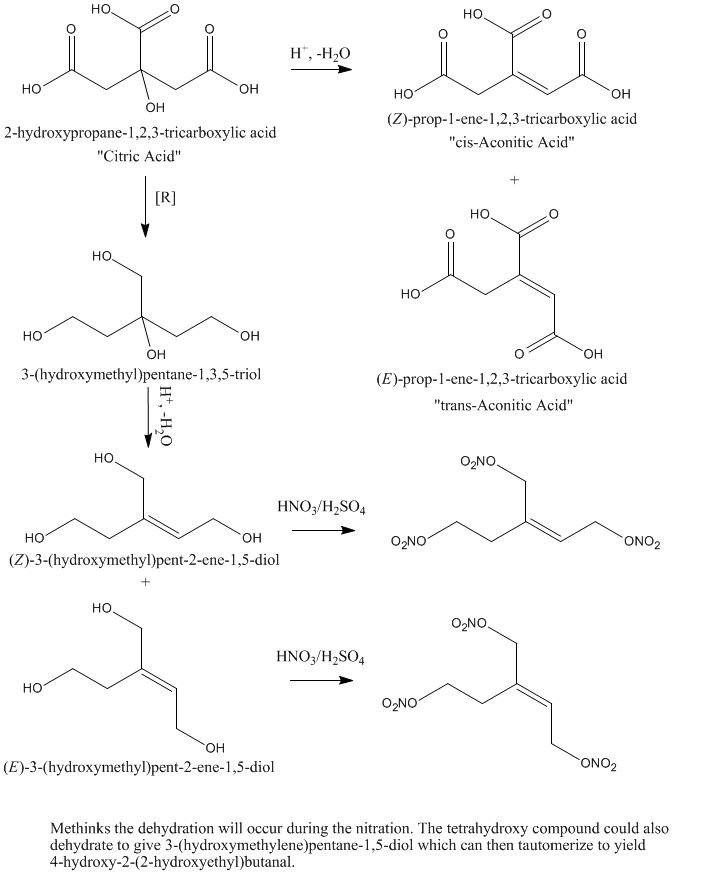
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Citric acid has also been the subject of fantasy for me for many cold nights, the fact that it has so many carboxyl groups is immediately seductive. I
also noticed that it has a fairly flexible structure, given a bit of prompting, I bet it could undergo some very interesting polymerization reactions.
For instance, I found it was a shame that peroxides are so unstable. Given the tendency for such a simple peroxide as acetone peroxide to form di- and
tetramers, surely 2-hydroxypropane-1,2,3-tricarbaldehyde would form some fascinating polymeric, crystalline and (relatively) stable peroxides?
Feel free to use http://www.sciencemadness.org/scipics/an_idea1.gif
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
You're going to waste ALOT of LiAlH4 if you're stupid enough to reduce citric acid directly. Not to mention the danger involved.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|