| Pages:
1
2 |
crushpack
Harmless

Posts: 9
Registered: 28-10-2004
Location: CZ
Member Is Offline
Mood: No Mood
|
|
colchicine
hi all isn't here anyone who has experience with colchicine extraction (from colchicum autumnale) to share it with the others?
I want the recipe for my friend who wants to use it in gardening but of course I'm interested too
Or if you find any good source on this extraction please let me know in this forum I would be most gratefull
thx
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
................see if you can get this, I can't get access to it maybe somone else can,.....solo
Supercritical carbon dioxide extraction of colchicine and related alkaloids from seeds of Colchicum autumnale L.
Ernesto Ellington, Jaume Bastida, Francesc Viladomat, Carles Codina *
Phytochemistry analysis vol.14, issue.3, pg.164
Department of Natural Products, Plant Biology and Edaphology, Faculty of Pharmacy, University of Barcelona, Barcelona, Spain
email: Carles Codina (ccodina@farmacia.far.ub.es)
*Correspondence to Carles Codina, Department of Natural Products, Plant Biology and Edaphology, Faculty of Pharmacy, University of Barcelona, Avda
Diagonal 643, Barcelona 08028, Spain
Keywords
Supercritical carbon dioxide extraction • colchicine • 3-demethylcolchicine • colchicoside • HPLC analysis • Colchicum autumnale
Abstract
A method for the extraction of the alkaloids colchicine, 3-demethylcolchicine and colchicoside from seeds of Colchicum autumnale by supercritical
carbon dioxide has been established. Several parameters such as pressure, temperature, percentage of modifier and extraction time have been examined.
Two extraction steps with constant carbon dioxide density (0.90 g/mL) and flux (1.5 mL/min) were required to extract the alkaloids in 110 min using 3%
methanol as modifier. The quantitative determination of the alkaloids was performed by HPLC; the percentages of recovery were higher than 98% for the
three alkaloids. This extraction procedure was compared with a conventional method involving maceration and sonication, and the same levels of
alkaloids were obtained in each case. The supercritical carbon dioxide method is, however, very efficient, more rapid and more environmentally
friendly than conventional methods. Copyright © 2003 John Wiley & Sons, Ltd.
Received: 30 November 2001; Revised: 5 February 2002; Accepted: 13 February 2002
Digital Object Identifier (DOI)
10.1002/pca.702 About DO
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Elawr
Hazard to Others
  
Posts: 174
Registered: 4-6-2006
Location: Alabama
Member Is Offline
Mood: vitriolic
|
|
Colchicine is readily available in pill form as a gout remedy. It is not a controlled substance, although in the US you have to have prescription from
a doctor. Extracting your own from the plant would be really cool though.
1
|
|
|
phangue
Harmless

Posts: 18
Registered: 1-10-2006
Member Is Offline
Mood: No Mood
|
|
Look for a copy of the U. S. DISPENSARY from the late 18-hundreds. There used to be one in our community collage library in this small N. Nevada city
until somebody ripped it off. Try a university library. These books contain detailed extractions for many alkaloids, including colchicine.
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Colchicine should be available OTC at pharmacies. According to the latest BNF, it is sold as 500mcg tables - net price for 20 tables £3.32. Not that
expensive.
Is the extraction possible with other forms of solvents, say the cheap dichloromethane?
phangue, I will try looking out for an old dispensary at med school. Thanks for the info. Will post here if I find anything. I guess there should be
something on the use of colchicum on Felter's Materia Medica, but don't think it goes so deep as to give an extraction.
(I missed it so much here I decided to make time to return. It's been a very long time since I posted here but I still used to visit every now and
then. Med school is though but I still love organic chemistry so darn much  ) )
Theory guides, experiment decides.
|
|
|
crushpack
Harmless

Posts: 9
Registered: 28-10-2004
Location: CZ
Member Is Offline
Mood: No Mood
|
|
Thanks for the supercritical carbon dioxide extraction method though I can't imagine how to perform it with my fairly limited home lab equipment
I would be more gratefull for some more conventional methods of extraction, typical for alkaloids, something like dissolving this substance in acid to
make a water soluble salt from it, which could be then simply extracted by water.
I want good yields and purity, both warranted, that is the reason why I think that experimentation (for which I have no time anyway) is not promising
me any success.
Moreover I would need some analytical (at least qualitative) method for this purpose - though of course if you know about any let me please know about
it
Maybe I should express my demand more accurately than before: I (resp. my friend of mine) want to gain colchicine from colchicum autumnale (my only
source) and <I>I don't care if it will be pure colchicine as a base or its salt</I> (though to be truth the last one possibility seems to
me better).
And of course to dispose with some analytical method for this substance would be pretty cool
Thank all for you help
[Edited on 24-11-2006 by crushpack]
[Edited on 24-11-2006 by crushpack]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
To attain biological small molecular water- and organic solvent-soluble compounds, I suppose a general purification routine can be used:
Crack cells (plants), with a blender and cellulase (washing powder contains that). Extract soluble compounds by centrifugation.
To remove soluble protein, dissolve up to 4M of ammonium sulphate (final concentration), and centrifuge (possibly filter) it off. The soluble fraction
should contain the colchicine, amongst a vast array of other compounds.
Now, since colchicine contains no free amine that can be converted to a salt, nor a free carboxy group, you can use that to remove anything containing
ionic compounds. For this, lyophilise (freezedry) the extract which has had its salt removed. Dissolve this in weak HCl, and extract the C. with
ether/DCM etc. To this extract, add NaOH (weak) and once again extract soluble compounds with ether/DCM. YOu should be left with an extract containing
organic nonionic, compounds, that lack COOH or free NH2 groups, devoid of peptides, carbohydrates and so on. So fairly pure. After this a
chromatography step will be necessary to isolate the final product (HPLC.... using i.e. an acetonitrile/water gradient).
It won't be easy, and you'll need decent equipment for a proper purification. But obtaining a reasonably pure extract should be possible with simple
methods.
May I ask, why are you after colchicine?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
dr. nick
Hazard to Self
 
Posts: 94
Registered: 20-6-2004
Location: Doh!land
Member Is Offline
Mood: yes
|
|
i don't know why he's after colchicine, but i am after it for use in plant breeding 
does there exist something like a approximate value how much plant mass of seeds will contain how much colchicine? Just asking, elseway one could
never guess the concentration of the solution one uses ...
thanks!
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
in German:
http://www.sciencemadness.org/talk/viewthread.php?tid=3564
http://www.sciencemadness.org/talk/viewthread.php?tid=6806
|
|
|
dr. nick
Hazard to Self
 
Posts: 94
Registered: 20-6-2004
Location: Doh!land
Member Is Offline
Mood: yes
|
|
wow, i'm flabbergasted (<-ahem ... can one say so?)
The rapidshare link is already dead, but the other one is awesome!
thanks a lot!
|
|
|
crushpack
Harmless

Posts: 9
Registered: 28-10-2004
Location: CZ
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
May I ask, why are you after colchicine? |
I want to use colchicine for the same purpose as dr. nick - plant breeding. But since I'm not gardener, in fact it's not me who want it but as I
already said the friend of mine. He wanted me to find out some extraction recipe and as reward I will have some chemical substance which I long sought
for - this is a good job i think
But of course being chemist the thing with the extraction is not without interest for me and if I will have some (more or less) pure colchicin in the
end the better - just for the pleasure of it if no other use occurs for it later.
Otherwise thanx for your proposal of extraction process maybe it can be of some use to me
|
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
I did few experiment with it,using aqueous sol. on seeds and on living specimens. Got at best 4 branch x internode which never expressed on the
offspring, due to the recessive nature of the gene itself. Also the growing females whith 4 branches and a peculiar squared stem would split in two
normal 2 opposite branch each internode or simply revert to normal.Good luck to you anyway!
|
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
X chemo. : it is said to stimulate mutants (polyploids:3 or 4 sets of genes instead of normal 2 present in a diploid).
Heavy stress does the same job eventually,nearly total stripping of a mature,fast growing plant for example, or any other extreme manipolation of the
enviromental parameter protracted overtime.This way would get a more permanent genetic mutation,as common sense suggest. If you have green thumbs,at 6
or more generation X year
it doesn't take long.That is BREEDING! [EUGenetic's ROCK!]
I have nae time nae space but if ... I would try recombinase carrier,maybe in cell culture first, then develop,select and cross, but this WEE human
breeding ties mae hands!
|
|
|
crushpack
Harmless

Posts: 9
Registered: 28-10-2004
Location: CZ
Member Is Offline
Mood: No Mood
|
|
Now when I've got some extraction methods it would be great to know somethink about its application. On my mind is especially its use in inducing
polyploidy in plants. Gil u said u have some experience with that I would be really gratefull if u can tell me more about it. U said to use it in
aequous solution and on seeds but could u give more details (f.e. how to make those solutions, how long to treat the seeds and so on)?
thx for your reply
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As this stuff is not a scheduled subatnce it ought to be commercially available and a lot cheaper than buying pharm grade in retail pill form.
Have a peak at Aldrich, Fluka, Merck, Acros, etc.
As for extraction, there is a book on alkaloids in the forum library (two volumes) available for free download. Doubtless it has a chapter on
colchicine. That will cover extraction, solublity, physical constants of the base and its salts etc etc. I am sure you will need the dried and
powdered (in a herb mill) plant and a lot of it is you expect to get a qty of the alkaloid. This is not a trivial process.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
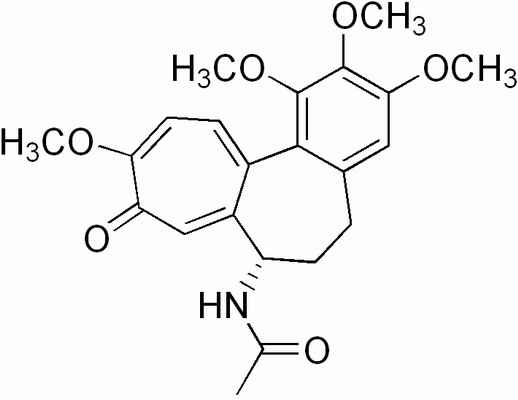
Here's the structure. This stuff is a noteworthy classical poison.
The structure strikes me as interesting. But as these fellows are only interested in plant breeding they will surely not be interested in hitting this
with permanganate to cleave those bonds in that six membered ring to give 3,4,5-trimethoxybenzaldehyde along with the 2,3,4 isomer, and some seven
membered trash to put out. No, I'm sure the thought never crossed their minds. Good botanists that they are.
[Edited on 17-2-2007 by Sauron]
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Colchicine is a reported mutagen, it create tetraploidy. This is why a lot of gardener tend to be interested in this substance, it create mutant plant
which are said to be stronger and bigger, and you can keep this tetraploidy over generation too, this first one being said to be a tad toxic due to
colchicine presence.
I would be very interested if you could develop the idea of hitting that with MnO4-. I'm still not a very good chemist and I don't see which bond
would be attacked and why, if anyone would care to explain to me I'd be very pleased.
[Edit]
Here are some propriety of Colchicine (Source: Merck 13th Edition):
Colchicine
Solubility:
1g dissolve in 22ml of water, 220ml ether and 100ml of benzene. Freely soluble in alcohol or chloroform. Praticaly insoluble in petroleum ether.
Melting Point: 142-150°C
It says here that it form a crystal compound with chloroform, the twon can be separated by heating for a long time at 60-70°C
And news refs to check:
Chemnitius, J. Prakt. Chem [II] 118, 29 (1928)
F. E. Hamerslag, Technology and Chemistry of Alkaloids (New York, 1950) pp. 66-80
[Edited on 17-2-2007 by Blind Angel]
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
How do you make phthalic acid? One way is to chew up napthalene with permanganate, most particularly a partially reduced napthalene and you end up
with only the two carboxyls hanging these where the second ring used to be.
Colchcine looks to be a setup for that sort of brute force oxidation. True it may be tricky to get the three methoxyl groups to survive in those
conditions. That may present a challenge.
Anyway maybe I am wrong and this is strictly botany and not chemistry, though I am not at all sure why toxicity is a concern in early generation
plants unless they are to be eaten...or perhaps, smoked.
I don't really care, it was all idle curiosity
|
|
|
albabatter
Harmless

Posts: 4
Registered: 20-2-2007
Location: Auld Reekie
Member Is Offline
Mood: emphatic
|
|
| Quote: | Originally posted by crushpack
Now when I've got some extraction methods it would be great to know somethink about its application. On my mind is especially its use in inducing
polyploidy in plants. Gil u said u have some experience with that I would be really gratefull if u can tell me more about it. U said to use it in
aequous solution and on seeds but could u give more details (f.e. how to make those solutions, how long to treat the seeds and so on)?
thx for your reply |
I would no bother unless outdoor.
The idea is to select 1000's seeds getting few mutants back,killing the rest in the process.Then monitor what genes mutated.Usually the interesting
ones are recessive.Take few bulbs,make a cold infusion,sprout your seeds in that .Most will die. Or try to wet the meristem after sprout every day,or
do it to cuttings.
The only polyploid commercial cultivar I remember of,are sicilian oranges,the cutting of which are fiercely guarded.I did not check but I suppose
Israeli may have cuttin edge study on the field. I prefer other way of breeding.
And as infusion goes, check(are you? or is Catanzaro?) salix (willow?). A bunch of young,growing stems in 1L. water is a very good root initiator (in
cuttings).Pseudomona putida is preferred as stimulator. Also, never judge a specimen until sure it reached full potential.
Extreme condition,manual defoliation,trimming,pruning will cause mutation too,as radical increase of U.V. levels will,also.
Chernobyl disaster did not mutate anything(in Liguria)
[Edited on 20-2-2007 by albabatter]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Colchicine, extracted from autumn crocus, has long been known as a cure for gout, in sub-toxic amounts. It works by dissolving the deposits of
crystals of uric acid ( 7,9-dihydro-1H-purine-2,6,8(3H)-trione, or C5H4N4O3, the final oxidation product of purine), in the tissues of extremities,
which cause the condition. The 7-membered unsaturated carbon ring with a keto oxygen and conjugated double bonds is a feature that it has in common
with derivatives made from atropine (from deadly nightshade) and cocaine (from coca), and this feature could have aromatic properties if separation of
charges resulting in the =O becoming -O- is energetically favored.
[Edited on by JohnWW]
|
|
|
magnus454
Hazard to Self
 
Posts: 57
Registered: 28-2-2007
Location: Clear Lake City, TX
Member Is Offline
Mood: No Mood
|
|
Probably get away with an ACETONE extraction of frozen and then ground up plant material, then evap. the acetone, and mix with distilled water,
anything water soluable should then dissolve out into the water, and you can then soak the seeds in it for the effect your wanting, try the MARIJUANNA
GROWERS GUIDE
History is repeating itself.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
JohnWW's post has the wrong structure for uric acid and the wrong mechanism for colchicine as a treatment for gout. There's more information here
http://en.wikipedia.org/wiki/Gout
While atropine does contain a 7 membered ring it is staurated, and atropine is the ester of an alcohol OH group attached to that ring rather than a
ketone. I think he's getting atropine muddled woth tropinone.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Tis doing this!
i ground three crocus "bulbs" added water and a little vinegar and let it sit for a few hours to dissolve. Filtered it, and let the smaller white
particles fall out.. i have a clear solution now
I soaked a Lettuce sprout in it for 20-40 minutes, hopefully it will make super lettuce 
i am also letting some other seeds soak, like morning glory and datura...
the morning glory is going to sit their for 24 hours, maybe only 15 or something idk and so will half the datura seeds, and then i will also put some
datura seeds in for about 5 hours
and compare and contrast germination rate, speed of growth, and size, i will also have just the normal seeds growing.
these seem to be the only seeds i can grow lol
i forgot to neutralize it by the way, ..so what effects will a little bit of acetic acid do to seeds? less then 1% I'm sure
but right, anyways my question is can you boil away water from colchicine?
i don't think its concentrated very much at all...
i know it decomposes from light so it makes me think that heat will do the same as well..
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Do you suffer from gout, KClO4? You could try taking the stuff to cure it, in sub-lethal amounts. Gout occurs in men who ingest both large amounts of
proteins, and acid-reacting foods and beverages especially alcohol. The testosterone in ordinary men causes the excessive retention in their bodies of
amino-acids from proteins, especially purine which oxidizes to uric acid.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Nope most people my age don't  doesn't that play a roll? doesn't that play a roll?
I dropped a few pill bugs in my solution, they started to die so it seemed, the color of their shell became a different grey, but they are still alive
for a while. this was ten to twenty minutes ago, and so far they seem to be getting better.
Perhaps they will do something cool if they live?
And i think, since its apparently fairly potent that my seeds are going to grow into some nice healthy polyploidys if i didn't kill them all.
and when my moss starts to grow i'm gonna add the colchicine to them and leave a control... might be rather interesting 
[Edited on 16-11-2007 by kclo4]
|
|
|
| Pages:
1
2 |