BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Weird copper compound
I'm rather puzzled by what happened when I decided to make copper compounds from pennies. I used HCl and H2O2, and when I added sodium hydroxide I got
an orange ppt the color of a pumpkin. I searched but couldn't find anything about an orange-colored copper compound, except maybe Cu2O, but the orange
compound was definitely distinct from that. I'm wondering what it was. Here's the whole procedure:
About 18 g of pennies (pre-1982: 95% Cu, 5% Zn) were cleaned with thiourea (tarn-x) and heated with 30 ml of 10 M HCl. About 20 ml of 3% H2O2 was
added in increments over a few hours. The solution initially quickly turned lime-green, and later became dark brown-green, with no ppts.
After cooling, the solution was neutralized and made basic by the addition of saturated NaOH which was contaminated with carbonate, but probably no
more than around 10%. An orange precipitate formed in the pH range of around 7 to 7.5. No blue copper hydroxide was ever observed. The orange solid
was vacuum filtered and washed, yielding several grams of extremely fine paste.
6 ml of 38% sulfuric acid was added to the orange solid, which immediately turned red-lavender and remained insoluble. The red solid is believed to
have been Cu2O (Copper(I)). Traces of blue sulphate could be seen, but virtually all red solid remained. 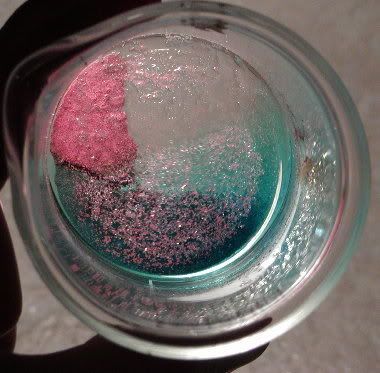 (IMAGE: oxide formed from orange solid with H2SO4. I did not get a picture of the orange compound itself.) (IMAGE: oxide formed from orange solid with H2SO4. I did not get a picture of the orange compound itself.)
Heating did not facilitate conversion of the red solid to sulfate. Addition of H2O2 did dissolve and convert all solid into blue solution with slight
effervescence.
Traces (~20 mg) of colorless crystalline material was filtered out. The solution was then reduced by boiling, and several grams of copper sulfate were
easily crystallized from the solution which contained a slight excess of H2SO4.
Copper(II) chloride can be reduced to copper(I) with HCl and Cu metal, which is basically the situation in the earlier part of my procedure. But CuCl
supposedly yields blue Cu(OH)2 with NaOH, and I do not think CuOH exists. Attempts were made to reproduce the orange substance. The same NaOH was used
to convert a sample of the obtained copper sulfate to deep-blue Cu(OH)2 with no problem. The hydroxide was made into a chloride mixture by adding HCl
and H2O2. This solution was re-basified with NaOH, with normal hydroxide again being obtained. Several other things were tried but I was never able to
reproduce the orange compound.
Whatever occured, I believe this is one way to make copper(I) oxide.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Bah, CuCl2 + OH --> Cu(OH,Cl)2 (at least in a green chloride complex solution), not Cu(OH)2.
In your case, the reduced brownness of the solution certainly made orange Cu2O. Cu2O disproportionates in H2SO4 to Cu metal (colloidial, having that
color) and Cu(II) in solution.
After soaking Cu metal in an acid solution for a while, you can get a nasty brown solution which hydrolyzes to white CuCl on dilution. This forms
orange Cu2O on neutralization, which disproportionates in acid as shown.
A reducing agent (like copper metal) added to your copper solution will give Cu(I) ions in part, which can be produced again in the same way.
Chloride ions and acid are required to dissolve the Cu(I), forming the brown complex. I'm not sure that Cu2O would be the first to precipitate on
neutralizing such a solution, but the rest of your experience seems to suggest Cu2O, showing that it is so.
Tim
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Thanks, 12AX7. So you're saying that the orange paste must have been the copper(I) oxide, and the red solid formed from that was copper. Ok, but I
have a few things which are befuddling me.
First, what do you mean by Cu(OH,Cl)2 ? Second, the photo on Wikipedia here sure looks alot like the red solid in my photo. Third, some of the red powder floats on the surface, which to me seems somewhat unlikely for
metallic copper even in colloidal form. Fourth, the red solid converted rather quickly into sulfate at room temperature, which metallic copper is
extremely slow to do, even with H2O2 (I know it had a much higher surface area).
I could be mistaken about those things. But I'm having trouble believing 100% that the red solid in the photo is copper. There's one way to find out,
but I'd rather not repeat the whole thing.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Cu(OH,Cl) is a basic chloride, containing both Cl- and OH- Think of it as a partially hydrolised chloride; you get basic salts of many acids such
as the 'sub' salts of bismuth.
Given that you also have some zinc in solution, you may get results that somewhat differ from using pure copper. When precipitating you might get
mixtures of zinc hydroxide or even oxychloride, which is somewhat resistent to not overly strong acid.
I've seen plenty of precipitates that had a bit floating on the surface of the solution, it can be quite annoying as the surface film may stick to
anything it touchs.
It does sound like crude Cu2O to me.
Next time use copper wire or pipe, avoid that 5% zinc.
[Edited on 4-11-2006 by not_important]
|
|
|
woelen
Super Administrator
        
Posts: 8041
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You made a copper (I) complex by dissolving your metal in HCl+H2O2. On adding NaOH you make (impure) hydrous Cu2O, which can have colors ranging from
yellow, brown to orange. I have done quite some experimenting with copper and wrote a few webpages about that. They may be helpful to you and explain
some of your observations:
http://woelen.scheikunde.net/science/chem/solutions/cu.html
http://woelen.scheikunde.net/science/chem/riddles/copperI+co...
|
|
|
BeerChloride
Harmless

Posts: 47
Registered: 17-9-2006
Location: Alabama, USA
Member Is Offline
Mood: Dunno
|
|
Thanks, woelen. I like the site. I was already wondering if perhaps both the orange and the red solids might in fact be different states of copper(I)
oxide! It seems to me that was the case.
And thanks, not_important. I do not believe I have heard of something like Cu(OH,Cl)2, at least not in that description. And I had already decided
wire or something would probably be better than pennies, although I think the zinc really did not present a problem.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Cu(OH,Cl)2 is the formula for copper oxychloride, better called hydroxychloride, produced by neutralizing a copper chloro- complex. The chlorides
don't entirely un-complex when neutralized, so the precipitate contains both hydroxyl and chloride. The proportion is indefinite (at least 20
varieties are known), so "(OH,Cl)2" is more apt than CuOHCl or something. This is green and does not decompose in hot water. Blue Cu(OH)2 decomposes
on heating as shown. Both decompose on dry heating, but the oxychloride disproportionates to CuO and CuCl2, which sublimates and decomposes easily,
giving off nasty fumes.
Tim
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
Just curious; Is that a marbles "bath" between the metal pot and what looks like a crockpot?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I was going to start a new thread in the Pyro/energetics area, Mods feel free to split this off if it`s a little Off Topic.
I`ve just finished what I believe to be CuCl2, it was simple copper carbonate in 30% HCl and added until the "fizzing" stopped and then a little drop
of excess HCl was added, now it`s been filtered to a nice clear blue/green soln.
time will tell upon drying if I`ve succeeded or not, as it should become a brownish powder when the .2H2O has been lost.
some sources say it`s used for a Blue color in pyro, others say green?
now upon heating to 1000c + CuCl2 = Cl + CuCl
is that single Chlorine enough to be an efficient chlorine donor, and also would I have to make the Composition Fuel rich as the Cl is an Oxidiser in
its own right?
I don`t have a whole load of this stuff only a few grams made for experiments sake, so I`de rather get it right First time with your advice 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
woelen
Super Administrator
        
Posts: 8041
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
When the solution is evaporated to dryness, then no brown CuCl2 will remain behind, but green impure CuCl2.2H2O. The green color will be due to
impurity, left over HCl in the product.
Once you have the green crystals, carefully heat (very slowly and constantly agitating, taking care of not getting the stuff too hot at once). When
heated very carefully, the water is driven off and brown CuCl2 remains behind. If the heating is too fast, then also HCl is driven off and then some
basic green oxychloride or hydroxychloride remains, Cu(OH/Cl)2 or Cu(O/Cl2). If the material becomes dirty green, then you've heated too strongly and
too fast. You can also test the purity of your CuCl2 by adding some to water. All of it should dissolve in excess of water without turbidity and with
a nice pale blue color.
CuCl2 gives a blue color in pyrotechnics. I did an experiment with this (but admittedly, combined with Cs-salts, so I'm cheating a bit  ): ):
http://woelen.scheikunde.net/science/chem/exps/barium_bromat...
|
|
|