| Pages:
1
2 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thanks. I compiled all the GIF images into a PDF file to make it more practical: Pericyclic reactions (Fleming, 1998)
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
That file still has some bugs in I need to weed out. I just wanted to see how much people want it before I take the extra effort.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Regarding the proposed aryne pathway, the problem is that when N-allylphtalimide is used, there is also a possibility of a proton abstraction from
the allylic amide, giving a species which could instead add to the aryne:
http://img349.imageshack.us/my.php?image=schemeop1.jpg
Question is if LDA is powerful enough to do this, it works for 2,3-wittig rearrangement*...
Don't know what the problem is but sometimes I can't show images...
IMO the original idea from p-benzoquinone is better, although acrylonitrile can be used to overcome this problem, other potential problems arise...
*: http://www.chempensoftware.com/reactions/rxn576.htm
Edit: Drunkguy, that's a cool book, please fix the bugs and upload it again...
[Edited on 3-11-2006 by Sandmeyer]
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Well, i would better said that the real problem with aryne pathway is side reactions between the base and the aryne in case of generation from
bromo-DMBenzene. When the aryne is done from the anthranylic zwiterion there is no base needed and less side reactino are possible (and the yiled
still suck to 20%). I think it may not work if another aryne generation pathway is used than from heating the zwitterion.
Also as nicodem pointed out, the acrylonitrile may add the base to its beta-carbon hence now i do not think it will work from bromo-DMB and
acrylonitrile neither. Also i do not think with an allylic non-conjugated to an EWG group it will add with the aryne as acrylonitrile did.
Problem with benzoquinone is the photochemistry may be abit tricky, do not forget than pyrex absorb UV light. Alltough i have no experience with
photochemistry at all i would like to know how to setup a good irradiation apparatus using a mercury HP lamp (solarium lamp)...
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
This is the new DL link:
http://www14.rapidupload.com/d.php?file=dl&filepath=8065
It still has a few narrow page margins, but it is readible.
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
I also uploaded a new book today.
The aryne mechanism is certainly valid with acylonitrile.
The small question remains as to how the hell one makes the appropriately functionalized aryne in the first place. Edit: My book covers some of these
methods. E.g. Treatment of monochloro-1,4-dimethoxybenzene with BuLi should result in aryne formation.
[Edited on 5-11-2006 by Drunkguy]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Drunkguy
The aryne mechanism is certainly valid with acylonitrile.
|
Are you saying this for the base formed aryne or the antranilic acid diazotation derived one? For the later we already know since that is how Nichols
did it. What is of issue here is whether the acrylonitrile would "survive" the LDA or NaNH2 needed for the aryne preparation from
2-bromo-1,4-dimethoxybenzene. (acrylonitrile tends to polymerize in the presence of strong bases like amides through the anionic polymerization
mechanism)
| Quote: | Originally posted by Drunkguy
The small question remains as to how the hell one makes the appropriately functionalized aryne in the first place. |
2-bromo-1,4-dimethoxybenzene is a piece of cake to prepare.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Uh, a 20% yield is got in that reaction although they dont comment on how the aryne was generated. However, in the further reading section they allude
to this journal article.
Also note that aryne generation can be accomplished by decomposition of diazonium salts -> aryl cation -> loss of proton = aryne.
|
|
|
Lego
Harmless

Posts: 8
Registered: 22-10-2005
Member Is Offline
Mood: No Mood
|
|
More superpotent agonists from Prof. Nichols
Molecular interaction of serotonin 5 HT2A receptor residues Phe339(6.51) and Phe340(6.52) with super-potent N-benzyl phenethylamine
agonists
Braden MR, Parrish JC, Naylor JC, Nichols DE
Mol. Pharmacol., 2006 http://dx.doi.org/10.1124/mol.106.028720
Abstract: Experiments were conducted to examine the molecular basis for the high affinity and potency of a new class of 5 HT2A
receptor agonists, N-benzyl phenethylamines. Competition binding assays at several serotonin receptors confirmed that an N-arylmethyl substitution was
necessary for affinity increases up to 300-fold over simple N-alkyl homologues, as well as enhanced selectivity for 5 HT2A vs. 5-HT2C and 5-HT1A
receptors. PI hydrolysis functional assays confirmed that these N-benzyl phenethylamines are potent and highly efficacious agonists at the rat 5 HT2A
receptor. Virtual docking of these compounds into a human 5 HT2A receptor homology model indicated that the N-benzyl moiety might be interacting with
F339((6.51)), whereas the phenethylamine portion was likely interacting with F340((6.52)). Experiments in h5 HT2A receptors with F339((6.51))L and
F340((6.52))L mutations appear to support this hypothesis. Dramatic detrimental effects on affinity, potency, and intrinsic activity were observed
with the F339((6.51))L mutation for all N-benzyl analogues, whereas most N-unsubstituted phenethylamines and traditional agonists were only weakly
affected, if at all. Consistent with other published studies, the F340((6.52))L mutation detrimentally affected affinity, potency, and intrinsic
activity of nearly all compounds tested, although a strong change in intrinsic activity was not seen with most N-aryl analogues. These data further
validate the topology of our h5 HT2A receptor homology model. Importantly, this study is the first to identify a hitherto unrecognized role for
residue 6.51 in agonist activation of a serotonin GPCR, whereas most previous reports have suggested a varied and sometimes contradictory role in
homologous GPCRs.
* Full details of chemical syntheses of all novel compounds and characterization of additional analogues will be described elsewhere
* By contrast, the N-benzyl group produced a profound increase in affinity (ca. 7- to 300-fold) and potency (ca. 100- to 200-fold) at the r5-HT2A
receptor. Addition of a polar methoxy or hydroxy group at the 2-position of the benzyl group further increased affinities
|
|
|
Lego
Harmless

Posts: 8
Registered: 22-10-2005
Member Is Offline
Mood: No Mood
|
|
C-(4,5,6-Trimethoxyindan-1-yl)methanamine: A Mescaline Analogue Designed Using a Homology Model of the 5-HT2A Receptor
Thomas H. McLean, James J. Chambers, Jason C. Parrish, Michael R. Braden, Danuta Marona-Lewicka, Deborah Kurrasch-Orbaugh, and David E.
Nichols
J. Med. Chem., 2006, 49(14), 4269-4274
http://dx.doi.org/10.1021/jm060272y

Abstract: A conformationally restricted analogue of mescaline, C-(4,5,6-trimethoxyindan-1-yl)-methanamine, was designed using a
5-HT(2A) receptor homology model. The compound possessed 3-fold higher affinity and potency than and efficacy equal to that of mescaline at the
5-HT(2A) receptor. The new analogue substituted fully for LSD in drug discrimination studies and was 5-fold more potent than mescaline. Resolution of
this analogue into its enantiomers corroborated the docking experiments, showing the R-(+) isomer to have higher affinity and potency and to have
efficacy similar to that of mescaline at the 5-HT(2A) receptor.
Experimental: 4,5,6-Trimethoxy-3H-indene-1-carbonitrile, 4. To a solution of 4,5,6-trimethoxyindanone[18] 3 (1.0 g, 4.5 mmol) in
CH2Cl2 (60 mL) under argon were added ZnI2 (40 mg) and trimethylsilyl cyanide (0.66 mL, 4.95 mmol). The mixture was heated at reflux for 5 h, and then
the solvent was evaporated. The resulting oil was redissolved in 40 mL of benzene, 0.5 g of Amberlyst-15 cation exchange resin was added, and the
flask was fitted with a Dean-Stark trap. The solution was heated at reflux for 3 h until no more water was collected. After filtration through
diatomaceous earth to remove the resin, the benzene was evaporated, and the resulting residue was chromatographed on silica (EtOAc/hexanes, 1:1) to
give 4 (0.71 g, 68%) as a yellow oil that solidified on standing: mp 95-97°C.
(±)-(2,3-Dihydro-4,5,6-trimethoxy-1H-inden-1-yl)aminomethane Hydrochloride, (±)-2. Unsaturated nitrile (±)-4 (1.40 g, 6.05 mmol) dissolved in
absolute EtOH (250 mL) was placed in a 500 mL glass Parr hydrogenation flask containing 250 mg of 5% Pd/C and shaken for 20 min under 20 psi of H2.
The solution was then filtered through diatomaceous earth to remove the catalyst and placed back into the hydrogenation flask along with 1 g of fresh
activated Raney nickel 2800. Ammonia gas was bubbled through the solution for 1 min, and the flask was then shaken under 45 psi of H2 for 6 h. The
catalyst was removed by filtration through diatomaceous earth, and the solvent was evaporated to yield a clear oil that was acidified with 1 N
methanolic HCl and evaporated to yield (±)-2 hydrochloride (1.58 g, 96%) as a white solid. An analytical sample was recrystallized from 95% EtOH (4.8
g, 87%): mp 245°C
18. Safir, S. R. Sedative Indanones. U.S. Patent 3,454,565, October 5, 1966 (American Cyanamid, Co)
http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=US3454565&...
[Edited on 1-12-2006 by Lego]
Attachment: J. Med. Chem., 2006, 49(14), 4269-4274.pdf (147kB)
This file has been downloaded 849 times
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by Lego
Molecular interaction of serotonin 5 HT2A receptor residues Phe339(6.51) and Phe340(6.52) with super-potent N-benzyl phenethylamine
agonists
Braden MR, Parrish JC, Naylor JC, Nichols DE
Mol. Pharmacol., 2006 http://dx.doi.org/10.1124/mol.106.028720
Abstract: Experiments were conducted to examine the molecular basis for the high affinity and potency of a new class of 5 HT2A
receptor agonists, N-benzyl phenethylamines. Competition binding assays at several serotonin receptors confirmed that an N-arylmethyl substitution was
necessary for affinity increases up to 300-fold over simple N-alkyl homologues, as well as enhanced selectivity for 5 HT2A vs. 5-HT2C and 5-HT1A
receptors. PI hydrolysis functional assays confirmed that these N-benzyl phenethylamines are potent and highly efficacious agonists at the rat 5 HT2A
receptor. Virtual docking of these compounds into a human 5 HT2A receptor homology model indicated that the N-benzyl moiety might be interacting with
F339((6.51)), whereas the phenethylamine portion was likely interacting with F340((6.52)). Experiments in h5 HT2A receptors with F339((6.51))L and
F340((6.52))L mutations appear to support this hypothesis. Dramatic detrimental effects on affinity, potency, and intrinsic activity were observed
with the F339((6.51))L mutation for all N-benzyl analogues, whereas most N-unsubstituted phenethylamines and traditional agonists were only weakly
affected, if at all. Consistent with other published studies, the F340((6.52))L mutation detrimentally affected affinity, potency, and intrinsic
activity of nearly all compounds tested, although a strong change in intrinsic activity was not seen with most N-aryl analogues. These data further
validate the topology of our h5 HT2A receptor homology model. Importantly, this study is the first to identify a hitherto unrecognized role for
residue 6.51 in agonist activation of a serotonin GPCR, whereas most previous reports have suggested a varied and sometimes contradictory role in
homologous GPCRs.
* Full details of chemical syntheses of all novel compounds and characterization of additional analogues will be described elsewhere
* By contrast, the N-benzyl group produced a profound increase in affinity (ca. 7- to 300-fold) and potency (ca. 100- to 200-fold) at the r5-HT2A
receptor. Addition of a polar methoxy or hydroxy group at the 2-position of the benzyl group further increased affinities |
Attachment: Molecular Interaction of Serotonin 5-HT2A Receptor Residues Phe339 (6.51) and Phe340(6.52) with Superpotent N-Benzyl Phe (247kB)
This file has been downloaded 836 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by Drunkguy
However, in the further reading section they allude to this journal article.
. |
HETARYNES
MANFRED G. REINECKE
Tetrahedron Vol. 38, No. 4, pp. 427,1982
http://mihd.net/m0fdt3
I. INTRODUCTION . . . . . . . . . . . . . , . . . . . . . .428
II. HISTORICAL PERSPECTIVE . . . . . . . . . . . . . . . . 428
III. SPECIAL PROBLEMS IN HETARYNE DETECTION . . . . . . 429
A. Cme Substitution
1. Normal substitution via arynes
2. Cine-substitution via transhalogenation (BCHD)
3. Cine- substitution via abnormal addiin-elimihtation (AEa)
4. Cine-substitution via addition-substitutiott-ehmination (A%)
5. Cine-substitution via addition-riag-openingcliminatian-ringclos~e (ANRORC tine)
6. Precautions
B. Cycloaddition
1. Dimerixation and trimerixation
2. Die&Alder reactions
3. Precautions
IV. SPECIAL PROBLEMS IN HETARYNE GENERATION . . . . . . . . 435
V. SURVEY OFHETARYNES. . . . , . . . . . . . . . . . . . . . . . . . .
A. Five-membered hetarynes
1. 2,3Didehydrobenxofuraa
2. Didehydrofurans
3. Didehydromaleic anhydride
4. 2,3-Didehydro-N-methylindole
5. Didehydropyrroles
6. Didehydromaleimide
7. Didehydrothiophenes
(a) Reactions which Rive typical aryne products by nonaryne mechanisms
(b) Reactions which failed to give aryne products
(c) Reactions which are speculated without evidence to give arynes
(d) Reactions which give aryne products probably via arynes
8. Didehydrothianapthenes
9. Didehydroselenophenes
10. Didehydroimidaxoles
11. Didehydropyraxoles
12. Didehydrothiaxole
13. Didehydroisothiaxoles
14. Didehydro-1,2,5-thiadiaxole
15. 1,2Didehydroazoles
16. Five-membered carbocyclic arynes
B. Six-membered hetarynes
1. 3,4Didehydrocoumarin
2. 3,4-Didehydropyridines
(a) Bidentate precursors
(b) Monodentate precursors
(c) Substituted 3,4didehydropyridines
3. 2,3-Didehydropyridii
(a) Bidentate precursors
(b) Monodentate precursors
4. Didehydfopyridixides
5. Other didehydropyridines
6. Didehydrodiies
(a) Didehydropyridazines
(b) Didehydropyrazines
(c) Didehydropyrimidines
7. Multicyclic didehydropyridines
(a) 3.CDidehydroquinolines
(b) 2,3_Didehydroquinolme
(c) 2,4-Didehydroquinoline
(d) Didehydroisoquinolines
(e) Didehydronaphthyridines
(f) Other bicyclic didehydropyridines
(g) Tricyclic Didehydropyridines
8. Multicyclic Didehydrodiazines
9. Didehydroborazine
C. Seven-membered hetarynes
D. Benzdidehydroheterocycles
Acknowledgement
VI. REFERENCES . . . . .
[Edited on 2-12-2006 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Panoramix
Harmless

Posts: 5
Registered: 14-2-2005
Member Is Offline
Mood: No Mood
|
|
Benzylation really helps
Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines
Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K
J. Med. Chem, 1994, 37(13), 1929-1935
Abstract: The effect of 15 different amine substituents on 5-HT2A and 5-HT2C serotonin receptor binding was investigated for two
series of compounds (i.e., phenylalkylamine and indolylalkylamine derivatives). In general, amine substitution decreases receptor affinity; however,
N-(4-bromobenzyl) substitution results in compounds that bind at 5-HT2A receptors with high affinity (Ki < 1 nM) and with > 100-fold
selectivity. Although parallel structural modification in the two series result in parallel shifts in 5-HT2C binding, these same modifications alter
5-HT2A binding in a less consistent manner.
One of the compounds, N-(4-bromobenzyl)-2CB, is 20 times more potent (Ki on 5-HT2A) than 2-CB
[Edited on by Panoramix]
Attachment: 4-Br-Benzyl-2CB.pdf (1MB)
This file has been downloaded 667 times
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
Perhaps, but I'd like to see how these N-benzylated substances are metabolized. It's well possible that the increased potency is nullified by a 100%
debenzylation before any drug reaches a receptor.
damnant quod non intelligunt
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
*Aminomethylbenzocycloalkanes *
Hello,
Sorry to dig up this old thread but I've just read it and i wonder if it can be possible to make the aminomethylbenzocycloalkane with
bromo-p-dimethoxybenzene and acrolein dimethylacetal via aryne formation. What do you think about that ?
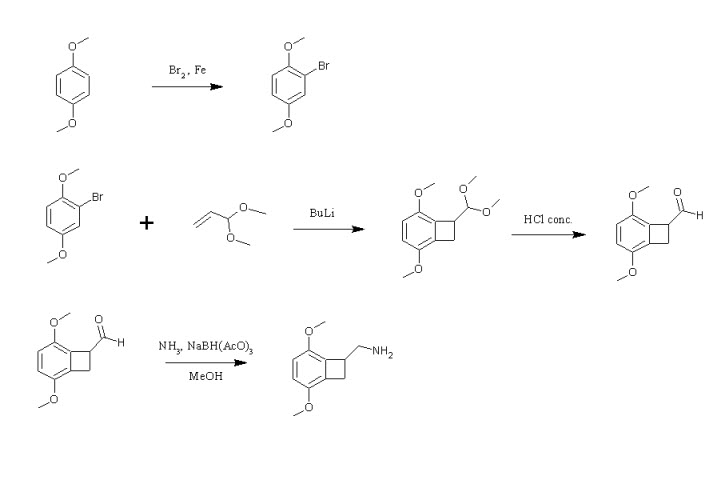
|
|
|
| Pages:
1
2 |
|