red_heat_cat
Harmless

Posts: 3
Registered: 16-10-2006
Member Is Offline
Mood: No Mood
|
|
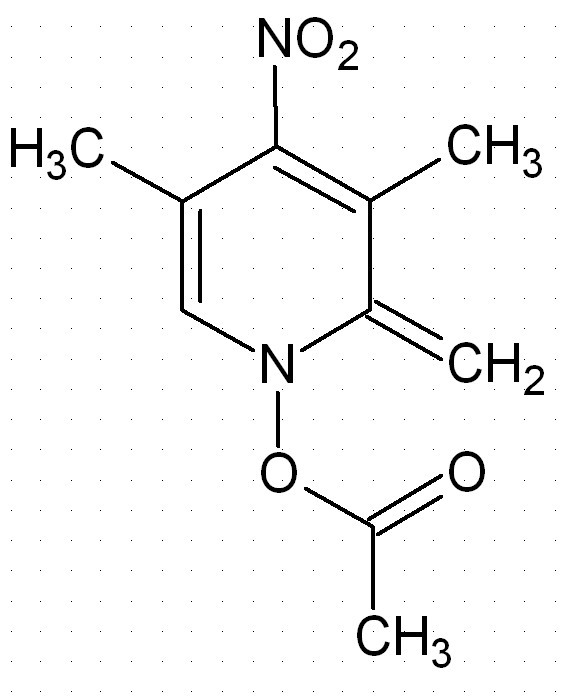
Is this an aromatic compound ?
Is this compound an aromatic? I don´t think so because there are 7 pi electrons in the ring. Is that right?
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
No, this is not an aromatic compound. It is not derived from a benzene ring (or a benzene ring with a C replaced by N).
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Neither is THF woelen but that's aromatic 
This molecule is not aromatic simply because it has no space for the third pi bond, the p orbital that is directed perpendicular to the plain is
already full, if it has been empty (if there had been a B there) the ring would have been aromatic.
[Edited on Mon/Oct/2006 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
THF is not aromatic.
Perhaps you meant Furan, Nerro?
[Edited on 16-10-2006 by garage chemist]
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
yes I did. (5 ring with 4 carbons and 1 nitrogen. between the carbons there are two double bonds.)
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Nerro, I indeed should have been more clear in my answer. I was referring to the benzene ring because of the six-membered ring in the molecule,
presented by red_heat_cat.
But aromaticity is a more general concept, e.g. the ion C5H5(-) also is aromatic, while it is not a six-membered ring. No classical model of this ion
can be written, 5 electrons are delocalized over the 5 C-atoms.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
not true, the ring has 4 + 2 electrons in its ring which complies with the rule.
C5H5- is a lot like pyrrole really, only nitrogen has a lone pair naturally while carbon only has it when its an anion.
[Edited on Mon/Oct/2006 by Nerro]
[Edited on Mon/Oct/2006 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Introductory Organic and Biochemistry -What does "aromatic" really mean?
http://www.geocities.com/athens/thebes/5118/obc/arom.htm
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Solo, thanks for that nice link. I again learnt something new and this is quite an interesting thing to learn and helps me understand things.
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
That compound is aromatic. By redrawing it like this it should become apparent. Hopefully this image displays properly:
[Edited on 17-10-2006 by Pyrovus]
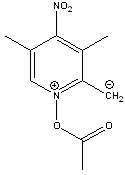
Never accept that which can be changed.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Thanks for clarifying this Pyrovus. Precisely my thinking. This should make a nicely reactive species. The NO2 group acts as an electron acceptor
(similar to picric acid) making this species more stable. Remember, styrene is also reactive, not only for polymerisation but also to form the
seperate charge species which are stabilised by resonance/pibonding.
Thought first this might be for 'Beginnings' but due to the discussion it'd might be just right here in org chem 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Separation of charges, in this instance to form a combined quaternary oxy-ammonium salt (two benzenoid resonance structures) and aliphatic carbanion,
takes a fair amnount of energy. To contribute to the overall structure, the overall gain in stability through this would have to be significant
compared to the other non-aromatic resonance structure shown at the start of this thread.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
| Quote: | Originally posted by JohnWW
Separation of charges, in this instance to form a combined quaternary oxy-ammonium salt (two benzenoid resonance structures) and aliphatic carbanion,
takes a fair amnount of energy. To contribute to the overall structure, the overall gain in stability through this would have to be significant
compared to the other non-aromatic resonance structure shown at the start of this thread. |
precisely my
thinking.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|