| Pages:
1
2
3 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Another idea to make CH2=O:
Make Ca formate, dry it:
Then heat it under a vigorous flame (the rector must be closed vs the flame and a cold trap has to be set on since aldehydes and cetons are very
flamable and risks of fire can result!!!):
Ca(O2CH)2 --> CaCO3 + CH2=O
BTW:the same procedure is was used to make aceton...CaCO3 can be reconverted into Ca carboxysalt by agitation with the acid (vinegar)
Ca(O2C-CH3) --> CaCO3 + CH3-CO-CH3
And if I remember wel:
Ca(O2C-CH3)2 + Ca(O2C-CH2-CH3)2 -->
CaCO3 + CH3-CO-CH3 + CH3-CO-CH2-CH3 + CH3-CH2-CO-CH2-CH3
So it is a convenient way to make aceton, MEK and diethyl ceton (3 pentanone).
A final aspect I think to remember is:
Ca(O2CH)2 + Ca(O2C-CH3)2 --> CH3-CO-CH3 + CH3-CH=O + CH2=O + CaCO3
Tath's all folks!
PH Z
|
|
|
plasma
Hazard to Self
 
Posts: 77
Registered: 20-5-2002
Location: Norway
Member Is Offline
Mood: Surprised
|
|
Thank's for the informative posts.
Let me see if I have understanded this correct. I mix 68% freezing cold 35% CH2O with 32% freezing cold conc NH4OH solution.
Then stir it for an hour and evaporate the liqiud at 40 C. Hexamine will crystalize.
BTW, I mentioned this procedure to a friend of mine and told him all I needed was formaldehyde. Supriseingly he had a 1 litre bottle of it in his
garage. (He is interested in biology and has used it for preserving plants).
I bought half a litre of him.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
You have almost understood everything but let's compare:
what you said:
"I mix 68% freezing cold 35% CH2O with 32% freezing cold conc NH4OH solution. Then stir it for an hour and evaporate the liquid at 40 C. Hexamine will
crystalize."
with what I said:
Add an excess of freezing cold NH4OH solution (max is 30% NH3 per weight) to a freezing cold one of CH2=O (formol/formaldehyde solution is max 40% by
weight over it deposit a precipitate of polyformal).Mix the two in stoechiometric amounts following the formula:
6CH2=O + 4NH3 --> C6H12N4 + 6H2O
Taking care to have a slight excess of NH3 solution of 20 to 30%!
Why the hell 68% CH2=O (35% solution) with 32% NH3 (32% solution)?
What is the stoechiometry I proposed you, where is the excess NH3?
You add an excess CH2O vs NH3; your cristallisation will thus contain some polyformal what is a big problem when brought in contact with HNO3 --> very
strongly exothermic runaway will occure!
Let see my explication deeper:
30% NH3 solution, means 300g of NH3/kg or 17,64 mole/kg solution!
40% CH2=O solution, means 400g of CH2=O/kg or 13,33 mole/kg solution!
Following the equation I have written:
6 moles of CH2O react with 4 moles of NH3!
Thus knowing that:
we have 17,64 mole/kg of NH3(30%), and 13,33 mole/kg of CH2O(40%); we need: 226,76g NH3 solution for 450,12g CH2O solution!
With the slight 25% excess of NH3 solution (to ensure 100% yield and no CH2O that is bad for further HNO3 exposure):
283,45g NH3 solution for 450,12g CH2O solution:
This makes 38,64% NH3 sol for 61,36% CH2O sol!
In you case you have 35% instead of 40% formol and 32 instead of 30% NH3; you will thus need:
450,12g*40/35= 514,43g CH2O solution and 283,45g*30/32= 265,73g NH3 sol.
Thus you would need:
34,1% NH3 solution(32%) for 65,9% CH2O solution or 341g/659g; you should get 179,5g Hexamine!
You were not far off but stil you might have had trouble with excess formol, better be cautious and not forget the 25% excess ammonia!
PH Z
|
|
|
plasma
Hazard to Self
 
Posts: 77
Registered: 20-5-2002
Location: Norway
Member Is Offline
Mood: Surprised
|
|
Made hexamine sucessfully today. I didn't have any conc NH3 solution left so I used 10% clear houshold ammonia instead. (adjusted the amounts) The
yeld was not as high as expected but satisfying.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The only reason why conc reactants are used is that they are already quite diluted if concentrated (30-40%) --> 70-60% water that has to be
evaporated!
PH Z
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
paraldehyde triperoxide
Since this topic is about organic peroxides, let's talk about paraldehyde peroxide. Paraldehyde are 3 polymerized acetaldehyde (ethanal) molecules
connected by the oxygen molecule of the aldehyde.
It is made by heating acetaldehyde with conc H2SO4.
Paraldehyde triperoxide would have the structure shown in the image. It might be very similar to AP, but the synthesis can't be, according to me
atleast. With AP the molecule ring is formed during peroxidation, here the peroxidation would take place after the ring closure,which seems quite
difficult. Or one could ofcourse try ring closure with H2O2 present.
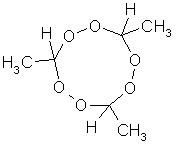
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I have attempted to prepare acetaldehyde peroxide using H2O2, acetaldehyde, and H2SO4. It failed, probably because the acetaldehyde was (this is my
hypothesis) just being oxidized to acetic acid. I didn't expect it to succeed, but what the hell, why not try...  I guess that failed experiment rules out the possibility of preparing formaldehyde peroxides. I guess that failed experiment rules out the possibility of preparing formaldehyde peroxides.
CH3CH(OH)(OOH) ----> CH3COOH + H2O
So the first step, reaction of CH3CHO with H2O2, results in the oxidation. I'm not sure if H2SO4 would be required for that reaction - I think that
CH3CHO would hydrate easily to CH3CH(OH)2, and so it would easily carry out a parallel reaction with H2O2 to form CH3CH(OH)(OOH)
I weep at the sight of flaming acetic anhydride.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Thanks
This is similar to the process I had seen, and overcomes the problem of excess oxidation of the CH2O, thanks a lot for posting.
|
|
|
Nevermore
Hazard to Others
  
Posts: 140
Registered: 3-5-2003
Location: China at the moment
Member Is Offline
Mood: shopping businessman
|
|
problems with AP synth
I'm having some strange troubles with AP synt using 3% h202 and pure acetone, i follow the recipe on megalomania's but i get no yeld at all.
The only changes i made are using 15ml acetone and 100ml h202, and then 7ml 40% h2so4. i cared the temp was always around 0, added slowly, stir in ice
and let it rest in fridge for 2 days but not even a cristal..also tried with HCL but no change..i start to suspect this h202 is no good since is
written "stabilised h2o2" maybe it has some compound that prevents the reaction to occurr? please advice any hint you think could be useful!
thanks!
Nevermore!
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
sorry
Acetone peroxide has been covered to death elsewhere. I've heard people claim success with 3% peroxide, but I think you would be much better off
with a higher concentration solution. Methods of H2O2 concentration are scattered about this forum and others.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Here some knowledge to a old new synthesis
by me to a meltable and thermic insensitive
dicyclic peroxide.
dicycloperoxide
It`s a fine powder, chemical stable and
insensitive against shock and high temperatures
with a sweet odour.
mp ~100 C
dc >350 C, (ignition)
ev ~ 7500 m/s
In convention with my ancestor.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Benzoyl Ketone + AP normal reaction?
Look like Benzoyl Peroxyde but with an added peroxo bridge
[Edited on 27-5-2003 by Blind Angel]
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Formaldehyde
I've seen CH<sub>2</sub>O produced by a rather powerful spark underwater using graphite electrodes.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
That's the bicyclic form of cyclohexanone peroxide.
I weep at the sight of flaming acetic anhydride.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I really think we should investigate cyclohexanedione peroxyde!
O=C(CH2-CH2)2C=O + 2H2O2 --> (HOO)(HO)C(CH2-CH2)2C(OH)(OOH)
This should lead to a relatively unsensitive polymer that has better OB than Aceton peroxyde.
(-O-O-)2C(CH2-CH2)2C(-O-O-)2C(CH2-CH2)C=
As I have proven many times, the higher the molecular weight the lower the sensitivity, the higher the melting point, the higher the density and the
better the VOD!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
And of course... nitro and dinitrocyclohexanone peroxydes
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
3% h202 should work just fine(ignoring the worthless yield,ofcourse).Chances are (with all the wank about terrorism and all)there was something in
you'r h202 to kill the reaction making AP.Perhaps if you'd warm to to a somewhat higher concentration and then repeat the experiment
we'd find out what's hapening.
Sorry for not havng anything to contribute as far as cyclohexanonan peroxide goes but a just a few questions:
<ol>
Was the spark in pure water,tap water or was there anything mixed with it?
Do you have any idea about the charge itself?
</ol>
|
|
|
Nevermore
Hazard to Others
  
Posts: 140
Registered: 3-5-2003
Location: China at the moment
Member Is Offline
Mood: shopping businessman
|
|
those experiments have been performed by pure water, tap water, with carbon rods, and by tungsten rods in saccarose solution. carbon is needed in the
reaction.
Nevermore!
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
more ...
heat citric acid to 100°C to make sure it's dehydrated. add conc sulfuric acid to get acetone dicarboxylic acid. I think this is the reaction:
H<sub>2</sub>SO<sub>4</sub> + HOOCCH<sub>2</sub>C(COOH)(OH)CH<sub>2</sub>COOH =>
HOOCCH<sub>2</sub>COCH<sub>2</sub>COOH + 2H<sub>2</sub>O + SO<sub>2</sub> + CO<sub>2</sub>
add the mix drop by drop to ice cold (preferably conc) H<sub>2</sub>O<sub>2</sub> <b>hoping</b> to get
di/tri/..mer of <b>per</b>acetone dicarboxylic acid peroxide. a cyclic dimer:
<img src="http://www.angelfire.com/rnb/pjff/padcp.gif">
C<sub>10</sub>H<sub>12</sub>O<sub>16</sub> => 10CO + 6H<sub>2</sub>O
Penguin Dictionary of Chemistry says phloroglucinol chemically behaves like both 1,3,5-trihydroxybenzene and triketone of cyclohexane(
>(-CH2-C=O-)3> ). now think about its peroxidation! (woW <img src=images/smilies/shocked.gif>
Edit: fixed the img
[Edited on 1-12-2003 by KABOOOM(pyrojustforfun)]
|
|
|
shadeT
Hazard to Self
 
Posts: 56
Registered: 3-8-2003
Member Is Offline
Mood: No Mood
|
|
does someone have some information about benzoyl peroxide ? i found some info on xinventions forum ... but nothing else ...
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 295
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
benzoyl peroxide
CAS number: [94-36-0]
melting point: 103 -106 °C (may explode when heated)
sparingly soluble in water or alcohol
soluble in benzene, chloroform, ether
made from: benzyol chloride and sodium peroxide
use: Source of free radicals for industrial processes. Oxidizing agent in bleaching oils, flour, etc.; catalyst in the plastics industry; initiator in
polymerization.
From the Merck Index
[Edited on 7-9-2003 by Mephisto]
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
Any ideas on the stability or power of methyl isobutyl ketone peroxide? I was thinking of ordering some methyl isobutyl ketone for the purpose of
making the peroxide of it but want to hear some thoughts about it before I bother ordering it.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
Krypton:
<blockquote>quote:<hr>Here some knowledge to a old new synthesis<BR>by me to a meltable and thermic insensitive<BR>dicyclic
peroxide.<BR><BR><a href="http://www.sciencemadness.org/scipics/dicycloperoxide.JPG"
target=_blank>dicycloperoxide</a><BR><BR>It`s a fine powder, chemical stable and <BR>insensitive against shock and high
temperatures <BR>with a sweet odour.<BR><BR>mp ~100 C <BR>dc >350 C, (ignition) <BR>ev ~ 7500
m/s<hr></blockquote>cyclohexanone peroxide is<b>n't</b>
C<sub>6</sub>H<sub>10</sub><(OO)2>C<sub>6</sub>H<sub>10</sub> it is<img
src="http://www.angelfire.com/rnb/pjff/chnp.gif">
from the condensed chemical dictionary:
<b>cyclohexanone peroxide</b> (1-hydroperoxycyclohexyl
1-hydroxycyclohexyl peroxide)
C<sub>6</sub>H<sub>10</sub>(OOH)OOC<sub>6</sub>H<sub>10</sub>OH.
Properties: Grayish paste; insoluble in water; soluble
in most organic solvents.
Hazard: Dangerous fire risk in contact with organic
materials. Strong oxidizing agent.
Shipping regulations: (Rail) (not over 50%) Organic
Peroxide label; (50-85% Organic Peroxide label.
Not acceptable passenger. (Air) (not over 50%)
Organic Peroxide label;(50-85%) Organic Peroxide
lable. Not acceptable passenger. (Over 85%) Not
acceptable.
7500 is too much. assuming it's quite less explosive than AP, I doubt its VOD to be higher than 5000 m/s
|
|
|
kleineskind
Harmless

Posts: 1
Registered: 13-12-2003
Location: USA
Member Is Offline
Mood: pacific
|
|
methyl isobutyl ketone
I found a local hobby shop that sells a lot of really cool chemicals. not in their chemistry section, but their painting section I found a container
of methyl isobutyl ketone. I plan on experimenting with it to see what kind of things can be formulated from it. making a peroxide may be
complicated due to the fact that there is little information if not none at all on how to procure such a substance. I enjoyed the method to create
hexamine. I plan on implementing the nitration of hexamine eventually. hexamine has been the most interesting substance to me. but I plan on mixing
methyl isobutyl ketone with many explosives so I can determine its application for mixtures. toluene was also available to me in its pure form at
this hobbyshop. let me just say that the best place to get chemicals is from frey scientific, but because they dont sell to people that do not belong
to a company or a school, the best places are 1- places that deal with photography, gardening, hobby shops, hardware stores, drug stores, paint shops,
and crafting. ebay has been my recent love. you can buy 8 pounds of potassium nitrate for a awesome price- $10.00!
\"A man\'s ethical behavior should be based effectually on sympathy, education, and social ties and needs; no religious basis is necessary.
Man would indeed be in a poor way if he had to be restrained by fear of punishment and hope of reward after death.\" - Albert Einstein
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
BTW, I've heard that dihydroperoxypropane could be formed using the common acetone/H2O2. I haven't done any search on it (I apologize if it
is the first link on google :p). Have someone tried it ?
It's oxygen balance (-70%) is far better from other peroxides such as the acetone one (di/tri or tetra meric) and DPPP and a bit betterthan HMTD
and TMDD. It's density is around 1.3 (following ACD/ChemSketch software).
But I'm sure with all these properties someone here had already tried it ! 
|
|
|
| Pages:
1
2
3 |