blindreeper
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Improvised still pics up!
I have managed to make a kind of still sorta thing. Made from flurecent tubes. It even has a viguerx column!
Still info and pics
I hope some of you hard core chemists find this interesting.
|
|
|
Basement Chemist
Hazard to Self
 
Posts: 92
Registered: 21-4-2003
Member Is Offline
Mood: underground
|
|
no i tried making a still out of home stuff and it sucked. the plastic coil condenser melted down and i had hot acid spilling out of a thin plastic
bottle. thanks alot! 
I am curently hiding from the DEA...
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
nice work! with some practise it is obvious that one can learn how to make
glass aparatus themselves.
but remember that fluoroscent tubes are made of soda glass, so it can take changes in temperature nearly as good as pyrex e t c. These glasses are
also much more difficult to work with since they have a much higher softening point.
just be careful and dont expect it to survive a 200+ C distillation unless being very careful. The thin walls helps to reduce temperature difference
between outside and inside of the glass.
I once tried to concentrate battery acid in a normal incandescent lightbuld, used as a round bottom flask. got it to about 300 C and heavy fuming.
Worked fine until I removed the heat and cold air got to it. Crack ! no fun, no good but I was prepared for it since I was curious about how it would
work.
Big halogen lamps are made of quarts or heat resistant glass, the one over 1000W become red hot in the glass after a few seconds!!
/rickard
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
looks good!
I rotated the pics for you (you have to use an image view/manipulation program to do this, like IrfanView). You get them here:
(right click, "save as"
- first picture
- second picture
Tell us how it works! And try it with something whats not expensive or hard to get and more important not dangerous first.
My stuff never works on first try  .... ....
|
|
|
blindreeper
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Thanks Organikum. Would any of you be interested in me making one with a proper condenser, vigurex column and a 135* angle still head plus a recovery
bend? (sorry no vacuum adaptors  ) )
I can start working on this on friday (get paid). I need to get some more tubes and some nichrome wire.
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
Note of Caution
Only dismantle fluorescent tubes in well ventilated areas, as they contain mercury vapour.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
I was wondering: Is the mercury vapor in a partial vacuum or is it near atmospheric pressure? Isolating it would be quite useful, taking the
necessary precautions, of course.
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
There is no mercury vapour in light tubes but a very small amount of metallic mercury (about 25miligram as I remember)
The white stuff lining the inside of the tube which turns the UV to visible light is more dangerous to health, so wearing gloves whilst brushing this
stuff (and the mercury) out and down the drain is advised. And no, it is no big sin against environment - the sewage handles this with easy. The
amounts are tiny the precautions are mainly directed to avoid as much unnecessary stress for the immune system as possible - the overall level is
already high enough.
No offense intended fish, only trying to debunk myths. In a world full of false warnings who will be able to recognize the real dangers and who will
listen to the serious warnings after this?
enough of seriosity - glassware is topic! What about this nice midscale RBF?
(no, not mine LOL....)

And this is actually mine (also I will again get accused to show off with glassware)
Thy FLUXISATOR (tm):

big magic!
  
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Damned.
This looks like I am trying to steal the show from blindreaper. Sorry - wasn´t intended.
I admit freely to be quite disabled in working with glass - some lighttubes straight - everything else broke.
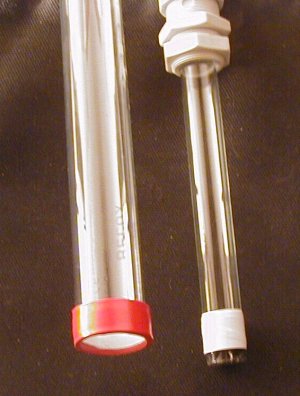
And thats it. I bow my head before blindreaper - you have talent for sure!
|
|
|
blindreeper
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
It is not a vacuum. The were gone long ago! They are now filled with an inert gas such as argon but for longer lasting bulbs they have krypton. I was
scared to shit the first time I tried to open a tube as I wasn't sure if it would implosed on me and then after getting on my afce sheild, gloves
and the tube was under several paices of cloth and in the box it came it. And then all I hear when it was penatrated is a slight sucking sound then
that was it. I was pissed indeed.
If you look on the box of a fluro tube they say "contains no mercury"
Well all of mine do 
Then again I think it's some barium compound that makes the "phosphor" inside the tube. Perhaps I should do a flame test? I also know
it's not soluable leading me to think it's barium sulfate...
I start work on the new still on friday so you should be seeing some pics sometime next week
|
|
|